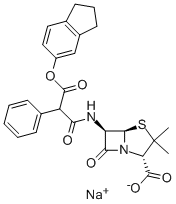
sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
- CAS NO.:21649-57-0
- Empirical Formula: C23H21N2NaO6S
- Molecular Weight: 476.47741
- EINECS: 244-496-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:35
![sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Structural](https://img.chemicalbook.in/CAS/GIF/21649-57-0.gif)
What is sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate ?
Description
Carbenicillin phenyl was synthesized by Beecham Research Laboratories in 1966 as an orally active carbenicillin. It is hydrolyzed to carbenicillin by intestinal esterase and thus acts the same when administered orally. The phenol produced by the hydrolysis is conjugated and excreted in urine.
Originator
Carfecillin sodium ,Beecham (GSK)
The Uses of sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
Antibacterial.
Definition
ChEBI: Carfecillin sodium is an organic sodium salt. It contains a carfecillin(1-).
Manufacturing Process
Phenylmalonic acid (27 g) was mixed with dry ether (80 ml) and treated with thionyl chloride (17.85 g, 10.9 ml) and dimethylformamide (4 drops). The mixture was refluxed for 3 hours on a hot water bath. The solvent was evaporated under reduced pressure and the residue dissolved in fresh dry ether (80 ml). Phenol (14.1 g) was added all at once and the mixture refluxed for 2 hours. The reaction was cooled to room temperature, washed with water (25 ml) and extracted with saturated sodium bicarbonate solution until the extracts were alkaline. The combined aqueous extracts were washed with ether (100 ml) and acidified with 5 N HCl. The precipitated oil was extracted with methylene chloride. The combined organic extracts were washed thoroughly with water (6x120 ml) dried over anhydrous magnesium sulphate and evaporated. The solid residue was crystallised from benzene to give monophenyl phenylmalonate, a colourless crystalline solid 30.2 g (78.7 %) MP 115-117°C. This product (5.12 g, 0.02 m) was mixed with thionyl chloride (20 ml) and heated in a water bath at 75°C for 1 hour. The excess thionyl chloride was evaporated under reduced pressure. The residue was mixed with dry benzene (10 ml) and again evaporated to dryness to remove residual thionyl chloride. The final residue was dissolved in dry acetone (100 ml) and added, with stirring, to a solution of 6-aminophenicillanic acid (4.32) in water (100 ml), 1 N sodium hydroxide (20 ml), 1 N sodium bicarbonate solution (30 ml) and acetone (50 ml) cooled to 12°C. The reaction mixture was stirred for 2 hours. The resulting mixture was stirred at room temperature for 2 hours. The resulting solution was extracted with ether (3x60 ml) and the extracts discarded. The aqueous layer was covered with ether (60 ml) and acidified with 1 N HCl to pH 2. The ether layer was separated and the aqueous layer extracted with ether (2x60 ml). The combine ether extracts were washed with water (20 ml) and extracted with 1 N sodium bicarbonate solution to pH 7. The neutral aqueous extract was evaporated under reduced temperature and pressure. The residue was dried over phosphorous pentoxide in vacuo to give 6.7 (70.4%) of penicillin salt as an amorphous solid. The solid, when dissolved in ethanol (50 ml) at room temperature gave on standing 30 min the penicillin salt as a colorless crystalline solid 5.23 g (78.1 %).
Therapeutic Function
Antibiotic
Properties of sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
| alpha | D20 +216.2° (H2O) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO:0.0(Max Conc. mg/mL);0.0(Max Conc. mM) |
Safety information for sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
Computed Descriptors for sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![sodium [2S-(2alpha,5alpha,6beta)]-6-[(1,3-dioxo-3-phenoxy-2-phenylpropyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate](https://img.chemicalbook.in/CAS/GIF/21649-57-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8