SILVER(II) FLUORIDE
Synonym(s):Silver difluoride
- CAS NO.:7783-95-1
- Empirical Formula: AgF2
- Molecular Weight: 145.87
- MDL number: MFCD00003411
- EINECS: 232-037-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
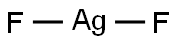
What is SILVER(II) FLUORIDE?
Chemical properties
dark brownish to dark grey powder
The Uses of SILVER(II) FLUORIDE
Silver(II) fluoride is used as a fluorinating and oxidizing agent. It is also used in the preparation of organic perfluorocompounds and in the fluorination of aromatic compounds. In addition, it is also used in pharmacy and electroplating industry. It serves as an intermediate in the catalysis of gaseous reactions with fluorine by silver. It is utilized in the oxidation of xenon to xenon difluoride.
The Uses of SILVER(II) FLUORIDE
In the fluorination of hydrocarbons.
What are the applications of Application
Silver(II) fluoride is a fluorinating agent
Safety Profile
Poison by subcutaneous route. Powerful oxidizing agent. hhxtures with boron + water are explosive. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and SILVER COMPOUNDS
Purification Methods
It is highly TOXIC because it liberates HF and F2. It is very hygroscopic and reacts violently with H2O. It is a powerful oxidising agent and liberates O3 from dilute acids, and I2 from I-solution. Store it in quartz or iron ampoules. It is white when pure; otherwise it is brown-tinged. It is thermally stable up to 700o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 241 1963.]
Properties of SILVER(II) FLUORIDE
| Melting point: | 690 °C(lit.) |
| Boiling point: | 700°C |
| Density | 4.57 g/mL at 25 °C(lit.) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in hexane, chloroform, chlorofluorocarbon and hydrogen fluoride. |
| form | Crystalline |
| color | Brown |
| Specific Gravity | 4.7 |
| Water Solubility | decomposed by H2O [CRC10] |
| Sensitive | Moisture Sensitive & Hygroscopic |
| Merck | 14,8513 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| CAS DataBase Reference | 7783-95-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silver fluoride (AgF2) (7783-95-1) |
Safety information for SILVER(II) FLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SILVER(II) FLUORIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Silver(II) fluoride CAS 7783-95-1View Details
Silver(II) fluoride CAS 7783-95-1View Details
7783-95-1 -
 Silver(II) fluoride CAS 7783-95-1View Details
Silver(II) fluoride CAS 7783-95-1View Details
7783-95-1 -
 Silver(II) fluoride CAS 7783-95-1View Details
Silver(II) fluoride CAS 7783-95-1View Details
7783-95-1 -
 Silver(II) fluoride 99% CAS 7783-95-1View Details
Silver(II) fluoride 99% CAS 7783-95-1View Details
7783-95-1 -
 Silver(II) fluoride CAS 7783-95-1View Details
Silver(II) fluoride CAS 7783-95-1View Details
7783-95-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
