Silicon dioxide
Synonym(s):Silica;Silicon dioxide;Silica gel;Quartz;SBA-15
- CAS NO.:7631-86-9
- Empirical Formula: O2Si
- Molecular Weight: 60.08
- MDL number: MFCD00148343
- EINECS: 231-545-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:07
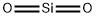
What is Silicon dioxide?
Chemical properties
white crystals or powder
Chemical properties
Diatomaceous earth is a transparent to gray, odorless amorphous powder.
Chemical properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
Physical properties
Colorless amorphous (i.e., fused silica) or crystalline (i.e., quartz) material having a low thermal expansion coefficient and excellent optical transmittance in far UV. Silica is insoluble in strong mineral acids and alkalis except HF, concentrated H3PO4, NH4 HF2 , concentrated alkali metal hydroxides. Owing to its good corrosion resistance to liquid metals such as Si, Ge, Sn, Pb, Ga, In, Tl, Rb, Bi, and Cd, it is used as crucible container for melting these metals, while silica is readily attacked in an inert atmosphere by molten metals such as Li, Na, K Mg, and Al. Quartz crystals are piezoelectric and pyroelectric. Maximum service temperature 1090°C.
The Uses of Silicon dioxide
Silicon(IV) oxide, amorphous is used as carriers, processing aids, anti-caking and free-flow agents in animal feed. Defoamer applications such as paint, food, paper, textile and other industrial applications. Synthetic silicon dioxides are used as a rheology control agent in plastics. It is also used to manufacture adhesives, sealants and silicones.
The Uses of Silicon dioxide
Silica (SiO2) (RI: 1.48) is mined from deposits of diatomaceous soft chalk-like rock (keiselghur). This is an important group of extender pigments, which is used in a variety of particle sizes. They are used as a flatting agent to reduce gloss of clear coatings and to impart shear thinning flow properties to coatings. They are relatively expensive.
Background
Silicon dioxide, or silica, is an oxide of silicon with the chemical formula SiO2. It is found in nature as agate, amethyst, chalcedony, cristobalite, flint, sand, QUARTZ, and tridymite as transparent and tasteless crystals. Inhalation of fine crystals is toxic to humans leading to respiratory toxicity. In powdered food products and pharmaceutical tablets, silicon dioxide is added as a flow agent to absorb water. Colloidal silica is also used as a wine, beer, and juice fining agent or stabilizer.
Definition
ChEBI: A silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens.
General Description
contains sodium stabilizing counterion
Hazard
Not toxic if ingested, inhaled silica dust can cause silicosis; carcinogen.
Agricultural Uses
Silica is silicon dioxide, one of the most abundant materials on the earth's crust. Quartz is an example of silica. It is used as a filler in fertilizers, and also, in the manufacture of glass, ceramics, abrasives, rubber and cosmetics.
Safety Profile
The pure unaltered form is considered a nuisance dust. Some deposits contain small amounts of crystahne quartz and are therefore fibrogenic. When diatomaceous earth is calcined (with or without fluxing agents) some sdica is converted to cristobalite and is therefore fibrogenic. Tridymite has never been detected in calcined batomaceous earth. See also other silica entries
Potential Exposure
Diatomaceous earth is used as a filtering agent and as a filler in construction materials, pesticides, paints, and varnishes. The calcined version (which has been heat treated) is the most dangerous and contains crystallized silica, and should be handled as silica. See also other entries on silica
Metabolism
Not Available
Shipping
This material is not singled out by DOT in its Performance-Oriented Packaging Standards.
Derivatives
Precipitated silica is obtained like silica gel by acidifying an aqueous solution of sodium silicate. Precipitated silica is used as filler in rubber for automobile tires and reinforcement particulate in elastomers, and as a flatting agent in paints and coatings for improving the flatness of coatings.
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.
Properties of Silicon dioxide
| Melting point: | >1600 °C(lit.) |
| Boiling point: | >100 °C(lit.) |
| Density | 2.2-2.6 g/mL at 25 °C |
| vapor pressure | 13.3hPa at 1732℃ |
| refractive index | 1.46 |
| Flash point: | 2230°C |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water and in mineral acids except hydrofluoric acid. It dissolves in hot solutions of alkali hydroxides. |
| form | suspension |
| pka | 6.65-9.8[at 20 ℃] |
| Specific Gravity | 2.2 |
| color | White to yellow |
| PH | 5-8 (100g/l, H2O, 20℃)(slurry) |
| Odor | at 100.00?%. odorless |
| Resistivity | 1∞10*20 (ρ/μΩ.cm) |
| Water Solubility | insoluble |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 6: forms irreversible hydrate |
| Crystal Structure | Trigonal |
| Merck | 14,8493 |
| Exposure limits | NIOSH: IDLH 3000 mg/m3; TWA 6 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 7631-86-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Silicon(iv) oxide(7631-86-9) |
| IARC | 3 (Vol. Sup 7, 68) 1997 |
| EPA Substance Registry System | Silica (7631-86-9) |
Safety information for Silicon dioxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Silicon dioxide
Silicon dioxide manufacturer
Prakash Chemicals International Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


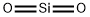

You may like
-
 Silica precipitated 98%View Details
Silica precipitated 98%View Details -
 Silica precipitated 98%View Details
Silica precipitated 98%View Details -
 Silica precipitated 98%View Details
Silica precipitated 98%View Details -
 Silica precipitated 99%View Details
Silica precipitated 99%View Details -
 Silica precipitated 98%View Details
Silica precipitated 98%View Details -
 Aerosil 98%View Details
Aerosil 98%View Details -
 Silica precipitated 99%View Details
Silica precipitated 99%View Details -
 Silicon dioxide, ≥99.9% CASView Details
Silicon dioxide, ≥99.9% CASView Details
