Silicon
- CAS NO.:7440-21-3
- Empirical Formula: H4Si
- Molecular Weight: 32.12
- MDL number: MFCD00085311
- EINECS: 231-130-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
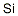
What is Silicon?
Chemical properties
grey lustrous solid or grey powder
Chemical properties
Silicon is a nonmetallic element which is known as silicon metal. Not occur freely in nature, but is found in silicon dioxide (silica) and in various silicates. It is a steel-gray crystalline solid or a black-brown amorphous material.
Physical properties
Silicon does not occur free in nature, but is found in most rocks, sand, and clay. Siliconis electropositive, so it acts like a metalloid or semiconductor. In some ways silicon resemblesmetals as well as nonmetals. In some special compounds called polymers, silicon will act inconjunction with oxygen. In these special cases it is acting like a nonmetal.
There are two allotropes of silicon. One is a powdery brown amorphous substance bestknown as sand (silicon dioxide). The other allotrope is crystalline with a metallic grayishluster best known as a semiconductor in the electronics industry. Individual crystals of siliconare grown through a method known as the Czochralski process. The crystallized siliconis enhanced by “doping” the crystals (adding some impurities) with other elements such asboron, gallium, germanium, phosphorus, or arsenic, making them particularly useful in themanufacture of solid-state microchips in electronic devices.
The melting point of silicon is 1,420°C, its boiling point is 3,265°C, and its density is2.33 g/cm3.
Isotopes
There are 21 isotopes of silicon, three of which are stable. The isotope Si-28makes up 92.23% of the element’s natural abundance in the Earth’s crust, Si-29 constitutes4.683% of all silicon found in nature, and the natural abundance of Si-30 ismerely 3.087% of the stable silicon isotopes found in the Earth’s crust.
Origin of Name
Silicon was named after the Latin word silex, which means “flint.”
Occurrence
Silicon, in the form of silicon dioxide (SiO2), is the most abundant compound in theEarth’s crust. As an element, silicon is second to oxygen in its concentration on Earth, yet it is only the seventh most abundant in the entire universe. Even so, silicon is used as the standard(Si = 1) to estimate the abundances of all other elements in the universe. For example, hydrogenequals 40,000 times the amount of silicon in the cosmos. Hydrogen is the most abundantof all elements in the universe, and carbon is just three and half times as abundant as siliconin the entire universe. On Earth silicon accounts for 28% of the crust, oxygen makes up 47%of the crust, and much of the rest of the crust is composed of aluminum.
It is believed that silicon is the product of the cosmic nuclear reaction in which alpha particleswere absorbed at a temperature of 109 Kelvin into the nuclei of carbon-12, oxygen-16,and neon-20. Pure elemental silicon is much too reactive to be found free in nature, but it doesform many compounds on Earth, mainly oxides as crystals (quartz, cristobalite, and tridymite)and amorphous minerals (agate, opal, and chalcedony). Elemental silicon is produced byreducing silica (SiO2) in a high-temperature electric furnace, using coke as the reducing agent.It is then refined. Silicon crystals used in electronic devices are “grown” by removing startercrystals from a batch of melted silicon.
History
Davy in 1800 thought silica to be a compound and not an element; later in 1811, Gay Lussac and Thenard probably prepared impure amorphous silicon by heating potassium with silicon tetrafluoride. Berzelius, generally credited with the discovery, in 1824 succeeded in preparing amorphous silicon by the same general method as used earlier, but he purified the product by removing the fluosilicates by repeated washings. Deville in 1854 first prepared crystalline silicon, the second allotropic form of the element. Silicon is present in the sun and stars and is a principal component of a class of meteorites known as “aerolites.” It is also a component of tektites, a natural glass of uncertain origin. Natural silicon contains three isotopes. Twenty-four other radioactive isotopes are recognized. Silicon makes up 25.7% of the Earth’s crust, by weight, and is the second most abundant element, being exceeded only by oxygen. Silicon is not found free in nature, but occurs chiefly as the oxide and as silicates. Sand, quartz, rock crystal, amethyst, agate, flint, jasper, and opal are some of the forms in which the oxide appears. Granite, hornblende, asbestos, feldspar, clay mica, etc. are but a few of the numerous silicate minerals. Silicon is prepared commercially by heating silica and carbon in an electric furnace, using carbon electrodes. Several other methods can be used for preparing the element. Amorphous silicon can be prepared as a brown powder, which can be easily melted or vaporized. Crystalline silicon has a metallic luster and grayish color. The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices. Hyperpure silicon can be prepared by the thermal decomposition of ultra-pure trichlorosilane in a hydrogen atmosphere, and by a vacuum float zone process. This product can be doped with boron, gallium, phosphorus, or arsenic to produce silicon for use in transistors, solar cells, rectifiers, and other solid-state devices that are used extensively in the electronics and space-age industries. Hydrogenated amorphous silicon has shown promise in producing economical cells for converting solar energy into electricity. Silicon is a relatively inert element, but it is attacked by halogens and dilute alkali. Most acids, except hydrofluoric, do not affect it. Silicones are important products of silicon. They may be prepared by hydrolyzing a silicon organic chloride, such as dimethyl silicon chloride. Hydrolysis and condensation of various substituted chlorosilanes can be used to produce a very great number of polymeric products, or silicones, ranging from liquids to hard, glasslike solids with many useful properties. Elemental silicon transmits more than 95% of all wavelengths of infrared, from 1.3 to 6.7 μm. Silicon is one of man’s most useful elements. In the form of sand and clay it is used to make concrete and brick; it is a useful refractory material for high-temperature work, and in the form of silicates it is used in making enamels, pottery, etc. Silica, as sand, is a principal ingredient of glass, one of the most inexpensive of materials with excellent mechanical, optical, thermal, and electrical properties. Glass can be made in a very great variety of shapes, and is used as containers, window glass, insulators, and thousands of other uses. Silicon tetrachloride can be used to iridize glass. Silicon is important in plant and animal life. Diatoms in both fresh and salt water extract silica from the water to build up their cell walls. Silica is present in ashes of plants and in the human skeleton. Silicon is an important ingredient in steel; silicon carbide is one of the most important abrasives and has been used in lasers to produce coherent light of 4560 ?. A remarkable material, first discovered in 1930, is Aerogel, which is now used by NASA in their space missions to collect cometary and interplanet dust. Aerogel is a highly insulative material that has the lowest density of any known solid. One form of Aerogel is 99.9% air and 0.1% SiO2 by volume. It is 1000 times less dense than glass. It has been called “blue smoke” or “solid smoke.” A block of Aerogel as large as a person may weigh less than a pound and yet support the weight of 1000 lbs (455 kg). This material is expected to trap cometary particles traveling at speeds of 32 km/sec. Aerogel is said to be non-toxic and non-inflammable. It has high thermal insulating qualities that could be used in home insulation. Its light weight may have aircraft applications. Regular grade silicon (99.5%) costs about $160/kg. Silicon (99.9999%) pure costs about $200/kg; hyperpure silicon is available at a higher cost. Miners, stonecutters, and other engaged in work where siliceous dust is breathed in large quantities often develop a serious lung disease known as silicosis.
Characteristics
The characteristics of silicon in some ways resemble those of the element germanium,which is located just below it in the carbon group.
Flint is the noncrystalline form of silicon and has been known to humans since prehistorictimes. When struck with a sharp blow, flint would flake off sharp-edged chips that were thenused as cutting tools and weapons.
In addition to silica (silicon dioxide SiO2), the crystal form of silicon is found in severalsemiprecious gemstones, including amethyst, opal, agate, and jasper, as well as quartz of varyingcolors. A characteristic of quartz is its piezoelectric effect. This effect occurs when thequartz crystal is compressed, producing a weak electrical charge. Just the opposite occurs whenelectric vibrations are fed to the crystal. These vibrations are then duplicated in the crystal.Quartz crystals are excellent timekeeping devices because of this particular characteristic.
The Uses of Silicon
In making silanes and silicones, the Si-C bond being about as strong as a C-C bond. In the manufacture of transistors, silicon diodes and similar semiconductors. For making alloys such as ferrosilicon, silicon bronze, silicon copper. As a reducing agent like aluminum in high tempereture reactions.
The Uses of Silicon
Silicon is usually available as electronic-grade, high-quality, high-purity single crystalline material in the form of wafers (round, surface-polished slices typically of 4–12 inches in diameter and a few hundreds of micrometers to millimeters in thickness). The biggest advantage of using silicon for microfluidic applications is the availability of a mature processing technology inherited from the microelectronics IC industry as well as the possibility of defining very small structures that can be cointegrated with the electronics on the same chip. Some of the disadvantages of using silicon as a structural material are linked to the polar nature of the silicon crystal resulting in undesirable adsorption of molecules in microfluidic systems. Furthermore, the higher cost of silicon as substrate material without any specific advantages from microfluidic systems standpoint makes it less attractive as a substrate material unless integration of on-chip electronic circuits is a strong requirement for the particular microsystem design. The typical cost of an average quality silicon substrate is about 0.25 U.S. cents/cm2.
The Uses of Silicon
silicone (volatile) is used in face creams to increase the product’s protection capabilities against water evaporation from the skin. Silicone polyethers are mainly used in water-based skin care formulations and give improved softness, gloss, and feel. Silicones have been used in cosmetics for more than 30 years. They are minerals able to repel water. Silicones present formulation problems because of poor compatibility with cosmetic oils and emollients. Silicones are not irritating.
The Uses of Silicon
Silicon’s tetravalent pyramid crystalline structure, similar to tetravalent carbon, results ina great variety of compounds with many practical uses. Crystals of silicon that have beencontaminated with impurities (arsenic or boron) are used as semiconductors in the computerand electronics industries. Silicon semiconductors made possible the invention of transistorsat the Bell Labs in 1947. Transistors use layers of crystals that regulate the flow of electric current.Over the past half-century, transistors have replaced the vacuum tubes in radios, TVs,and other electronic equipment that reduces both the devices’ size and the heat produced bythe electronic devices.
Silicon can be used to make solar cells to provide electricity for light-activated calculatorsand satellites. It also has the ability to convert sunlight into electricity.When mixed with sodium carbonate (soda ash) and calcium carbonate (powdered limestone)and heated until the mixture melts, silica (sand) forms glass when cooled. Glass ofall types has near limitless uses. One example is Pyrex, which is a special heat-resistant glassthat is manufactured by adding boron oxide to the standard mixture of silica, soda ash, andlimestone. Special glass used to make eyewear adds potassium oxide to the above standardmixture.
Silicon is also useful as an alloy when mixed with iron, steel, copper, aluminum, andbronze. When combined with steel, it makes excellent springs for all types of uses, includingautomobiles.
When silicon is mixed with some organic compounds, long molecular chains known assilicone polymers are formed. By altering the types of organic substances to these long siliconepolymer molecules, a great variety of substances can be manufactured with varied physicalproperties. Silicones are produced in liquid, semisolid, and solid forms. Silicones may berubbery, elastic, slippery, soft, hard, or gel-like. Silicone in its various forms has many commercialand industrial uses. Some examples are surgical/reconstructive implants, toys, SillyPutty, lubricants, coatings, water repellents for clothing, adhesives, cosmetics, waxes, sealants,and electrical insulation.
Background
Silicon is under investigation in clinical trial NCT00103246 (Photodynamic Therapy Using Silicon Phthalocyanine 4 in Treating Patients With Actinic Keratosis, Bowen's Disease, Skin Cancer, or Stage I or Stage II Mycosis Fungoides).
Definition
Nonmetallic element Atomic number 14, group IVA of the periodic table, aw 28.086, valence = 4, three stable isotopes. It is the second most abundant ele- ment (25% of the earth’s crust) and is the most important semiconducting element; it can form more co
Definition
silicon: Symbol Si. A metalloid element belonging to group 14 (formerlyIVB) of the periodic table; a.n.14; r.a.m. 28.086; r.d. 2.33; m.p.1410°C; b.p. 2355°C. Silicon is thesecond most abundant element inthe earth’s crust (25.7% by weight) occurring in various forms of silicon(IV)oxide (e.g. quartz) and in silicateminerals. The element is extractedby reducing the oxide with carbon inan electric furnace and is used extensivelyfor its semiconductor properties.It has a diamond-like crystalstructure; an amorphous form alsoexists. Chemically, silicon is less reactivethan carbon. The element combineswith oxygen at red heat and isalso dissolved by molten alkali. Thereis a large number of organosiliconcompounds (e.g. siloxanes) althoughsilicon does not form the range ofsilicon–hydrogen compounds andderivatives that carbon does (seesilane). The element was identifiedby Antoine Lavoisier in 1787 andfirst isolated in 1823 by J?ns Berzelius.
Reactions
Si is a little soluble in water (Solubility: 0.005 g/100 g H2O (298 K)). It reacts strongly with F at room temperature, Cl at 430℃, Br at 500℃, O at 400℃, and N2 at 1000℃ to form compounds of SiF4 , SiCl4 , SiBr4 , SiO2 , SiO, and Si3N4 , respectively.
General Description
A dark brown powder. Insoluble in water and denser than water. Burns readily when exposed to heat or flames, and may be difficult to extinguish. Water may not be effective in extinguishing flames. Used to make computer microchips.
Air & Water Reactions
Highly flammable. Insoluble in water. A significant dust explosion hazard.
Reactivity Profile
Silicon is a reducing agent. Ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. Burns spontaneously in gaseous chlorine. A mixture of silicon, aluminum, and lead oxide explodes when heated [Mellor 7:657 1946-47]. When heated with an alkali carbonate, a vigorous reaction attended by incandescence occurs [Mellor 6:164 1946-47]. Reacts violently with silver fluoride [Mellor 3:389 1946-47]. Reacts with sodium-potassium alloy to form sodium silicide, which is spontaneously flammable in air [Mellor 2 Supp. 2:564 1961].
Hazard
Flammable in powder form.
Hazard
The dust of silicon oxide (silicate) can burn or explode and is very harmful if inhaled.Continued exposure to silica dust causes silicosis, a form of pneumonia.
The hydrides of silicon (silicon plus hydrogen) are extremely volatile and spontaneouslyburst into flames in air at room temperatures. They must be kept in special vacuum chambers.
Over the past several decades, there has been some concern over the potential hazards andsafety of the cosmetic use of silicone body implants—breast implants, in particular. Severalmanufactures have been sued over the failure of the implants, and the federal government (FDA) withdrew its approval for their use. Congressional hearings with manufacturers in2005 produced new information that has reversed the FDA’s ban on their use—but only withcertain manufacturers of implants. The debate continues.
Health Hazard
Oxides from metallic fires are a severe health hazard. Inhalation or contact with substance or decomposition products may cause severe injury or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
May react violently or explosively on contact with water. Some are transported in flammable liquids. May be ignited by friction, heat, sparks or flames. Some of these materials will burn with intense heat. Dusts or fumes may form explosive mixtures in air. Containers may explode when heated. May re-ignite after fire is extinguished.
Flammability and Explosibility
Non flammable
Agricultural Uses
Some plants need silicon (Si) in addition to micro and
macro nutrients. It is one of the most abundant elements
absorbed by plants. Silicon belongs to Group 14
(formerly IVB) of the Periodic Table. It is an
essential trace element for the normal growth of higher
animals, as it is involved in the formation of bones and
cartilages. Crops grown in the absence of soluble silica
are more prone to mildews than those provided with
soluble silica. Rice, cucumber, gherkin and barley
require silicon. Silicon improves the growth of sugar
cane. Silicon corrects soil toxicities arising from the
presence of excessive quantities of Mn, Fe and active Al.
The oxidizing power of rice roots and their tolerance to
the high level of iron and manganese are attributed to
silicon nutrition.
In a field trial, when parts of a rice crop had a silica to
nitrogen ratio of 11:2, 10 tons/ha of extra rice was
produced. Silicon helps to (a) maintain the erectness of
rice leaves, (b) increase resistance to insect pests, (c)
improve photosynthesis, (d) increase the number of
stems, and (e) improve the fresh as well as dry weights of
rice plants. If silica is withheld during the reproductive
period, the number of spikelets per panicle and the
ripened grain percentage decreases.
Silicon contributes to the structure of cell wall, thus
(a) making the cell wall more immune to diseases, (b)
improving the stalk strength, and (c) increasing the
resistance to lodging. Rice and sugar cane respond
favorably to silicon fertilizers. Enzyme-silicon
complexes formed in sugar cane act as protectors or
regulators of photosynthesis and enzyme activity. By
suppressing the invertase activity in sugar cane, silicon
increases sugar production.
Silicon and oxygen occupy 75% of the earth's crust,
of which silicon alone is 27.7%. Silica, which occurs to
the extent of 60 to 80% as insoluble quartz silica, is in the
form of mono-silicic acid [Si(OH)4], the availability of
which increases with increasing soil pH and temperature.
Roughly, 130 ppm silicon (SO2) is the critical limit of the
available silicon in air-dry soil for maximizing wetland
rice yield; the critical limit is raised by adding silicon (as
SiO2).
Silicon uptake by plants differ with plant species.
Gramineae contains 10 to 20 times more silicon than that normally found in legumes and dicotyledons. Paddy
contains 4.6 to 7.0% of silicon in the straw. Oxides of
iron and aluminum, liming, flooding and nutrient supply
influence silicon-uptake; high soil-water content
increases the uptake in rice, barley, oats, sorghum and
sugar cane.
The quantity of silica fertilizer to be added to the soil
is guided by the ratio of the available silica to organic
matter, which if less than 100, warrants the use of
fertilizer; if the ratio is more than 100, it calls for the
addition of organic matter; if less than 50, it indicates that
the soil is suffering from silicon shortage. For a silicondeficient
area, the addition of 2 todha of silica fertilizer
is recommended. Freckling, which is a necrotic leaf spot
condition, is a symptom of low levels of silicon in a sugar
cane plant that receives direct sunlight, the ultraviolet
(W) radiation in sunlight being the causative agent.
Sufficient quantities of silicon in a sugar cane plant filters
out harmful UV radiation.
Major silica fertilizers include calcium silicate slag,
calcium silicate and sodium meta-silicate. 1.5 to 2.0 tons
of silicate slag per hectare usually provides sufficient
silicon for rice crops produced in low-silicon soils.
Silicate fertilizers serve as a source of silicon and a liming
material in acid soils. Slags from the steel industry,
ground basic-slag (containing varying quantities of Al,
Ca, Fe, Mn, Mg and Si), and wollastonite (Ca-Mg
silicate) are all silicate fertilizers.
Sodium silicate increases the crop yield in phosphatedeficient
soils, possibly because silicates help increase
the assimilation of phosphoric acid by the plant and not
the soil. However, according to some, silicate increases
the amount of available soil phosphate. Heavy
applications of nitrogen make the rice plant more
susceptible to fungal attack because of the decreased
silicon concentration in the straw.
Industrial uses
Silicon is, after oxygen, the second most abundant element in the earth s crust. It occurs in a range of minerals and sand (SiO2, quartz). Silicon can be extracted from silicates or sand by reducing SiO2 with coke at high temperatures at around 3000°C.Silicon is used in a wide variety of applications. In nature, silicon does not exist as the pure metal and most commonly occurs in silica (including sand) and silicates. Silicon dioxide, also known as silica, is a hard substance with a high melting temperature and clearly very different from carbon dioxide. Molten silica can be used to make glass, an extremely useful material, which is resistant to attack by most chemicals except fluorine, hydrofluoric acid and strong alkalis. Silicon atoms can also be found in the class of compounds called silicones. Pure silicon metal is used in semiconductors, the basis of all electronic devices, and is most well known for its application in solar panels and computer chips.
Safety Profile
A nuisance dust. Moderately toxic by ingestion. An eye irritant. Does not occur freely in nature, but is found as sdicon dioxide (sdtca) and as various shcates. Elemental Si is flammable when exposed to flame or by chemical reaction with oxidlzers. Violent reactions with alkali carbonates, oxidants, (A1 + PbO), Ca, Cs2C2, Cl2, CoF2, F2, IFs, MnF3, Rb2C2, FNO, AgF, NaK alloy. When heated it will react with water or steam to produce H2; can react with oxidizing materials. See also various silica entries, SILICATES, and POWDERED METALS.
Potential Exposure
Silicon may be used in the manufacture of silanes, silicon tetrachloride, ferrosilicon, silicones. It is used in purified elemental form in transistors and photovoltaic cells.
Metabolism
Not Available
Shipping
UN1346 Silicon powder, amorphous requires, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Incompatibilities
Dust or powder may form explosive mixture with air. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, calcium, carbonates, chlorine, fluorine, oxidizers, cesium carbide; alkaline carbonates.
Properties of Silicon
| Melting point: | 1410 °C(lit.) |
| Boiling point: | 2355 °C(lit.) |
| Density | 2.33 g/mL at 25 °C(lit.) |
| storage temp. | Flammables area |
| solubility | insoluble in H2O, acid solutions; soluble in alkaline solutions |
| form | powder |
| color | White |
| Specific Gravity | 2.42 |
| Odor | Odorless |
| PH | 13.5 (H2O, 20°C) |
| Water Solubility | INSOLUBLE |
| Sensitive | Air Sensitive |
| Crystal Structure | Cubic, Diamond Structure - Space Group Fd3m |
| Merck | 13,8565 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Dielectric constant | 2.4(Ambient) |
| Stability: | Stable. Fine powder is highly flammable. Incompatible with oxidizing agents, bases, carbonates, alkali metals, lead and aluminium oxides, halogens, carbides, formic acid. |
| CAS DataBase Reference | 7440-21-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silicon(7440-21-3) |
| EPA Substance Registry System | Silicon (7440-21-3) |
Safety information for Silicon
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Silicon
| InChIKey | BLRPTPMANUNPDV-UHFFFAOYSA-N |
Silicon manufacturer
Palmo Industrial Silicones Pvt. Ltd.
Silicon Products (P) Associates
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Silicon granular, Virgin poly fines CAS 7440-21-3View Details
Silicon granular, Virgin poly fines CAS 7440-21-3View Details
7440-21-3 -
 Silicon granular, Virgin poly fines CAS 7440-21-3View Details
Silicon granular, Virgin poly fines CAS 7440-21-3View Details
7440-21-3 -
 Silicon granular, Virgin poly fines CAS 7440-21-3View Details
Silicon granular, Virgin poly fines CAS 7440-21-3View Details
7440-21-3 -
 Silicon CAS 7440-21-3View Details
Silicon CAS 7440-21-3View Details
7440-21-3 -
 Silicon CAS 7440-21-3View Details
Silicon CAS 7440-21-3View Details
7440-21-3 -
 Silicon CAS 7440-21-3View Details
Silicon CAS 7440-21-3View Details
7440-21-3 -
 Silicon standard solution CASView Details
Silicon standard solution CASView Details -
 Silicon standard solution CASView Details
Silicon standard solution CASView Details