Barium titanate
Synonym(s):Barium metatitanate;Barium titanate (IV);Barium titanium oxide;Titanium barium oxide
- CAS NO.:12047-27-7
- Empirical Formula: BaO3Ti
- Molecular Weight: 233.19
- MDL number: MFCD00003447
- EINECS: 234-975-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 17:18:48
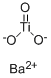
What is Barium titanate?
Description
Attributing to its ferroelectricity, barium titanate is applied as a semiconductor with positive temperature coefficient of resistivity. It is one of the most important electroceramic materials among all the ferroelectric materials. Barium titanate has also dielectric properties, which can be used in the manufacture of ceramic capacitors. Furthermore, barium titanate has piezoelectric property and can be used in electrical devices such as actuators, accelerators, ultrasonic generators, piezoelectric transducers, filters, and sensors.
Chemical properties
White crystalline solid; exists in five crystal modifications; the common tetragonal form has a Curie point of 120°C; exhibits ferroelectric and piezoelectric properties; density 6.02 g/cm3; melts at 1,625°C; insoluble in water and alkalies; slightly soluble in dilute mineral acids; dissolves in concentrated sulfuric acid and hydrofluoric acid.
Physical properties
White crystalline solid; exists in five crystal modifications; the common 94 BARIUM TITANATE tetragonal form has a Curie point of 120°C; exhibits ferroelectric and piezoelectric properties; density 6.02 g/cm3; melts at 1,625°C; insoluble in water and alkalies; slightly soluble in dilute mineral acids; dissolves in concentrated sulfuric acid and hydrofluoric acid.
Physical properties
Energy Gap:
Eg=3:38 eV(E//c); 3:27 eV(E⊥c).
The energy gap decreases by -4.5×10-4 eV/℃ when the crystal structure becomes cubic at high temperature.
Dielectric Constants:
Saturated Polarizability: 78,000 esu, Dipole Moment: 5×10-18 esu cm-3 .
The Uses of Barium titanate
Barium titanate has many important commercial applications. It has both ferroelectric and piezoelectric properties. Also, it has a very high dielectric constant (about 1,000 times that of water). The compound has five crystalline modifications, each of which is stable over a particular temperature range. Ceramic bodies of barium titanate find wide applications in dielectric amplifiers, magnetic amplifiers, and capacitors. These storage devices are used in digital calculators, radio and television sets, ultrasonic apparatus, crystal microphone and telephone, sonar equipment, and many other electronic devices.
The Uses of Barium titanate
In electronic devices, e.g., as voltage-sensitive dielectric in so-called dielectric amplifiers, in computer elements, magnetic amplifiers, memory devices.
The Uses of Barium titanate
Barium titanate (BaTiO3, BT) is one of the widely used materials in electronic ceramics. Because of its very high permittivity, it could be used in capacitors with outstanding properties by doping. Because barium titanate is used in large quantities as a material in electronic devices, considerable energy savings could be achieved if barium titanate could be sintered at a reduced temperature. It is reported that for LTCC (Low Temperature Cc-fired Ceramics) application, barium titanate is sintering at 1000℃ for 24 hours with the incorporation of silicate glass system. In addition, there is a report saying that a sintered body of relative density around 90% was achieved by 900℃ for 8 hours by adding boron oxide or lead borate to a barium titanate.
Preparation
Barium titanate is made by sintering a finely powdered mixture of barium carbonate and titanium dioxide in a furnace at 1,350°C. The calcined mass is finely ground and mixed with a binder (plastic). The mixture is subjected to extrusion, pressing or film casting to obtain ceramic bodies of desired shapes. Plastic is burnt off by heating and the shaped body is sintered by firing and then polished.
Barium titanate also may be prepared by other methods. These include ignition of barium and titanium alcoholates in an organic solvent; treatment of tetraethyl titanate or other alkyl ester of titanium with an aqueous solution of barium hydroxide; and ignition of barium titanyloxalate.
General Description
Barium titanate(IV) is a ceramic material that can be synthesized by reacting titanium oxide (TiO2) and barium salts in the presence of oleic acid as a stabilizing agent. It has a cubic crystal structure which allows it to have ferroelectric, thermoelectric and piezoelectric properties. It forms a film with intense photoluminescence which can be used for a wide range of electronic applications.
Structure and conformation
The space lattice of BaTiO 3 takes four types of crystal systems, depending on temperatures. The trigonal crystal (lower than -90℃) shows the spontaneous polarization to the [111] direction.The rhombic crystal (-70℃ to 5℃) shows the spontaneous polarization to the [011] direction and takes a longer structure along the direction. The tetragonal crystal (5℃–120℃) shows the spontaneous polarization to the [001] direction and takes a longer structure along the direction by the ratio of c/a=1.01. The cubic crystal (higher temperature than Curie point 120℃) takes the calcium titanate structure (Perovskite structure) and Ba atoms position at the eight corners of the cube, six O atoms at the center of the planes, and a Ti atom at the center of the body.
References
[1] Burcu Ertu?, The Overview of The Electrical Properties of Barium Titanate, American Journal of Engineering Research, 2013, vol. 02, 01-07
[2] G. Arlt, D. Hennings and G. de With, Dielectric properties of fine‐grained barium titanate ceramics, Journal of Applied Physics, 1985, vol. 58, 1619
[3] Tomoaki Karaki, Kang Yan and Masatoshi Adachi, Barium Titanate Piezoelectric Ceramics Manufactured by Two-Step Sintering, Japanese Journal of Applied Physics, 2007, vol. 46, 7035-7038
Properties of Barium titanate
| Melting point: | 1625 °C |
| Density | 6.08 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | no restrictions. |
| solubility | Soluble in alcohols |
| form | powder |
| Specific Gravity | 6.08 |
| color | White to gray |
| PH | 9.6 (20g/l, H2O, 25℃)(slurry) |
| Water Solubility | INSOLUBLE |
| Merck | 14,1000 |
| CAS DataBase Reference | 12047-27-7(CAS DataBase Reference) |
| EPA Substance Registry System | Barium titanium oxide (BaTiO3) (12047-27-7) |
Safety information for Barium titanate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Barium titanate
| InChIKey | WNKMTAQXMLAYHX-UHFFFAOYSA-N |
Barium titanate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
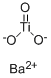
You may like
-
 BARIUM TITANATE 99%View Details
BARIUM TITANATE 99%View Details -
 Barium titanium oxide CAS 12047-27-7View Details
Barium titanium oxide CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide CAS 12047-27-7View Details
Barium titanium oxide CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide, High surface area CAS 12047-27-7View Details
Barium titanium oxide, High surface area CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide, High surface area CAS 12047-27-7View Details
Barium titanium oxide, High surface area CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
12047-27-7 -
 Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
Barium titanium oxide, Polymeric precursor CAS 12047-27-7View Details
12047-27-7
