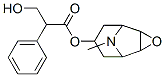
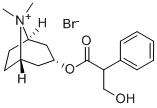
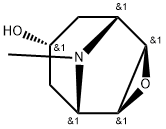
Scopolamine hydrobromide
Synonym(s):(−)-Scopolamine hydrobromide trihydrate;Hyoscine hydrobromide;Scopine tropate;Scopolamine hydrobromide
- CAS NO.:114-49-8
- Empirical Formula: C17H21NO4.BrH
- Molecular Weight: 384.26
- MDL number: MFCD00012647
- EINECS: 204-050-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
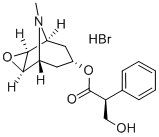
What is Scopolamine hydrobromide?
Description
Scopolamine is a tropane alkaloid that can be found in many plants of the Solanaceae (nightshade) family. It is a muscarinic receptor antagonist that can be used to induce memory impairment in animals. Scopolamine prevents motion sickness, nausea, and vomiting in animals.
Chemical properties
Off-White Solid
The Uses of Scopolamine hydrobromide
An acetylcholine antagonist. Used in treatment of motion sickness; antiemetic; antispasmodic; mydriatic; preanesthetic medicant
What are the applications of Application
(?)-Scopolamine hydrobromide is a competitive inhibitor of acetylcholine muscarinic receptors
Definition
ChEBI: A hydrobromide that is obtained by reaction of scopolamine with hydrogen bromide.
brand name
Isopto Hyoscine (Alcon); Transderm-Scop (Ciba-Geigy).
General Description
Colorless crystals or white powder or solid. Has no odor. pH (of 5% solution): 4-5.5. Slightly efflorescent in dry air. Bitter, acrid taste.
General Description
Scopolamine hydrobromide(hyoscine hydrobromide) occurs as white orcolorless crystals or as a white, granular powder. It is odorlessand tends to effloresce in dry air. It is freely soluble inwater (1:1.5), soluble in alcohol (1:20), only slightly solublein chloroform, and insoluble in ether.
Scopolamine is a competitive blocking agent of theparasympathetic nervous system as is atropine, but it differsmarkedly from atropine in its action on the higher nervecenters. Both drugs readily cross the blood-brain barrierand, even at therapeutic doses, cause confusion, particularlyin the elderly.
Air & Water Reactions
Sensitive to air, light and moisture. Water soluble.
Reactivity Profile
Scopolamine hydrobromide is incompatible with acids, bases and oxidizing agents. .
Fire Hazard
Flash point data for Scopolamine hydrobromide are not available; however, Scopolamine hydrobromide is probably combustible.
Biological Activity
Non-selective muscarinic antagonist. Widely used clinically to treat motion sickness.
Clinical Use
A sufficiently large dose of scopolamine will cause an individualto sink into a restful, dreamless sleep for about8 hours, followed by a period of approximately the samelength in which the patient is in a semiconscious state.During this time, the patient does not remember events thattake place. When scopolamine is administered with morphine,this temporary amnesia is termed twilight sleep.
Metabolism
Hyoscine hydrobromide is almost entirely metabolised, probably in the liver; only a small proportion of an oral dose is excreted unchanged in the urine. In one study in man, 3.4% of a single dose, administered by subcutaneous injection was excreted unchanged in urine within 72 hours.
storage
Store at RT
Purification Methods
The hydrobromide is recrystallised from Me2CO, H2O or EtOH/Et2O and dried. It is soluble in H2O (60%) and EtOH (5%) but insoluble in Et2O and slightly in CHCl3. The hydrochloride has m 300o (from Me2CO). The free base is a viscous liquid which forms a crystalline hydrate with m 59o and [] D 20 -28o (c 2.7, H2O). It hydrolyses in dilute acid or base. [Meinwald J Chem Soc 712 1953, Fodor Tetrahedron 1 86 1957, Beilstein 6 III 4185.]
Properties of Scopolamine hydrobromide
| Melting point: | 195-199 °C (dry matter)(lit.) |
| alpha | D25 -24 to -26° (c = 5, calculated on anhydrous basis) |
| storage temp. | Store at RT |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 114-49-8(CAS DataBase Reference) |
| EPA Substance Registry System | Scopolamine hydrobromide (114-49-8) |
Safety information for Scopolamine hydrobromide
Computed Descriptors for Scopolamine hydrobromide
Scopolamine hydrobromide manufacturer
Prism Industries Ltd
Related products of tetrahydrofuran
You may like
-
 114-49-8 Scopolamine hydrobromide 98%View Details
114-49-8 Scopolamine hydrobromide 98%View Details
114-49-8 -
 Scopolamine hydrobromide 99%View Details
Scopolamine hydrobromide 99%View Details
114-49-8 -
 Scopolamine hydrobromide 95% CAS 114-49-8View Details
Scopolamine hydrobromide 95% CAS 114-49-8View Details
114-49-8 -
 Scopolamine Hydrobromide CAS 114-49-8View Details
Scopolamine Hydrobromide CAS 114-49-8View Details
114-49-8 -
 Hyoscine hydrobromide CASView Details
Hyoscine hydrobromide CASView Details -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8