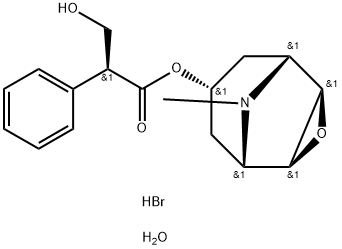
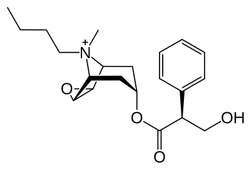
Scopolamine butylbromide
Synonym(s):(−)-N-Butylscopolamine bromide;(−)-Scopolamine N-butyl bromide;Hyoscine N-butyl bromide
- CAS NO.:149-64-4
- Empirical Formula: C21H30BrNO4
- Molecular Weight: 440.37
- MDL number: MFCD00078561
- EINECS: 205-744-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:18:46
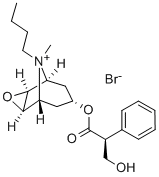
What is Scopolamine butylbromide?
Chemical properties
Crystalline Solid
Originator
Butylscopolamine,China Pharm
The Uses of Scopolamine butylbromide
Anticholinergic. Antispasmodic
The Uses of Scopolamine butylbromide
(?)-Scopolamine N-butyl bromide was used as standard in designing a procedure for quantification of compounds using CE-MS.8
What are the applications of Application
N-Butylscopolammonium Bromide is an anticholinergic and antispasmodic
Definition
ChEBI: Butylscopolamine bromide is an organic bromide salt of butylscopolamine. It is an antispasmodic drug which can relieve painful stomach cramps (including those linked with irritable bowel syndrome), bladder and menstrual cramps. It has a role as a muscarinic antagonist and an antispasmodic drug. It is an organic bromide salt and a quaternary ammonium salt. It contains a butylscopolamine.
Manufacturing Process
1300 g of scopolamine base and 350 g of n-butylbromide in 600 ml acetonitrile is heated at 65°C for 160 hours. The oil obtained is dissolved in methanol. The solution is cooled and crystalline scopolamine N-n-butylbromide is filtered. After recrystallization from methanol was obtained scopolamine N-n-butylbromide with melting point 142-144°C and [α]d 20 = -20.5° (3% solution in water); yield 65%.
Therapeutic Function
Anticholinergic, Spasmolytic, Antitussive
Biochem/physiol Actions
Competitive muscarinic acetylcholine receptor antagonist; antispasmodic.
Clinical Use
Symptomatic relief of gastrointestinal or genitourinary
disorders due to smooth muscle spasm
Bowel colic
Excessive respiratory secretions
Metabolism
The main metabolic pathway is the hydrolytic cleavage of the ester bond. Orally administered hyoscine butylbromide is excreted in the faeces and in the urine. Studies in man show that 2-5% of radioactive doses is eliminated renally after oral, and 0.7-1.6% after rectal administration. Approximately 90% of recovered radioactivity can be found in the faeces after oral administration. The urinary excretion of hyoscine butylbromide is less than 0.1% of the dose. The metabolites excreted via the renal route bind poorly to muscarinic receptors and are therefore not considered to contribute to the effect of the hyoscine butylbromide.
Properties of Scopolamine butylbromide
| Melting point: | 142-1440C |
| alpha | D20 -20.8° (c = 3 in water) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white |
| optical activity | [α]25/D 20.8°, c = 3 in H2O(lit.) |
| CAS DataBase Reference | 149-64-4(CAS DataBase Reference) |
Safety information for Scopolamine butylbromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Scopolamine butylbromide
Scopolamine butylbromide manufacturer
Bazayan & Co.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Hyoscine butylbromide 99%View Details
Hyoscine butylbromide 99%View Details -
 Hyoscine butylbromide 149-64-4 98%View Details
Hyoscine butylbromide 149-64-4 98%View Details
149-64-4 -
 149-64-4 98%View Details
149-64-4 98%View Details
149-64-4 -
 Hyoscine butylbromide CAS 149-64-4View Details
Hyoscine butylbromide CAS 149-64-4View Details
149-64-4 -
 Hyoscine butylbromide CAS 149-64-4View Details
Hyoscine butylbromide CAS 149-64-4View Details
149-64-4 -
 (−)-Scopolamine N-butyl bromide CAS 149-64-4View Details
(−)-Scopolamine N-butyl bromide CAS 149-64-4View Details
149-64-4 -
 Hyoscine Butylbromide EP Impurity D, 25mgView Details
Hyoscine Butylbromide EP Impurity D, 25mgView Details
1229224-94-5 -
 Hyoscine Butylbromide API Powder, PrescriptionView Details
Hyoscine Butylbromide API Powder, PrescriptionView Details
149-64-4
