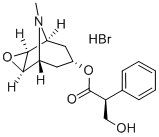
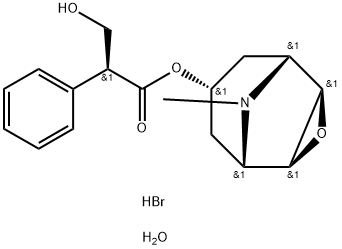
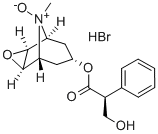
Scopolamine
Synonym(s):KIAA0606;PHLPP;PHLPP1;PLEKHE1;SCOP
- CAS NO.:51-34-3
- Empirical Formula: C17H21NO4
- Molecular Weight: 303.35
- MDL number: MFCD05662373
- EINECS: 200-090-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-19 16:27:22
What is Scopolamine?
Absorption
The pharmacokinetics of scopolamine differ substantially between different dosage routes. Oral administration of 0.5 mg scopolamine in healthy volunteers produced a Cmax of 0.54 ± 0.1 ng/mL, a tmax of 23.5 ± 8.2 min, and an AUC of 50.8 ± 1.76 ng*min/mL; the absolute bioavailability is low at 13 ± 1%, presumably because of first-pass metabolism. By comparison, IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a Cmax of 5.00 ± 0.43 ng/mL, a tmax of 5.0 min, and an AUC of 369.4 ± 2.2 ng*min/mL.
Other dose forms have also been tested. Subcutaneous administration of 0.4 mg scopolamine resulted in a Cmax of 3.27 ng/mL, a tmax of 14.6 min, and an AUC of 158.2 ng*min/mL. Intramuscular administration of 0.5 scopolamine resulted in a Cmax of 0.96 ± 0.17 ng/mL, a tmax of 18.5 ± 4.7 min, and an AUC of 81.3 ± 11.2 ng*min/mL. Absorption following intranasal administration was found to be rapid, whereby 0.4 mg of scopolamine resulted in a Cmax of 1.68 ± 0.23 ng/mL, a tmax of 2.2 ± 3 min, and an AUC of 167 ± 20 ng*min/mL; intranasal scopolamine also had a higher bioavailability than that of oral scopolamine at 83 ± 10%.
Due to dose-dependent adverse effects, the transdermal patch was developed to obtain therapeutic plasma concentrations over a longer period of time. Following patch application, scopolamine becomes detectable within four hours and reaches a peak concentration (tmax) within 24 hours. The average plasma concentration is 87 pg/mL, and the total levels of free and conjugated scopolamine reach 354 pg/mL.
Toxicity
Scopolamine overdose may manifest as lethargy, somnolence, coma, confusion, agitation, hallucinations, convulsion, visual disturbance, dry flushed skin, dry mouth, decreased bowel sounds, urinary retention, tachycardia, hypertension, and supraventricular arrhythmias. In some cases, overdose symptoms may appear similar to those associated with withdrawal following discontinuation. However, withdrawal symptoms such as bradycardia, headache, nausea, abdominal cramps, and sweating can help to distinguish between these possibilities. Overdose management primarily involves the removal of all transdermal patch systems combined with symptomatic and supportive care. Ensuring an adequate airway, supplemental oxygen, establishing intravenous access, and continuous monitoring are recommended. In cases where patients have swallowed one or more patch systems, it may be necessary to remove them or administer activated charcoal.
Animal studies revealed an oral LD50 of 1880 mg/kg in mice and 1270 mg/kg in rats, and a subcutaneous LD50 of 1650 mg/kg in mice and 296 mg/kg in rats.
Description
Scopolamine?(1)?and its biochemical precursor hyoscyamine?(2)?are deadly-nightshade alkaloids that are also found other plants of the Solanaceae family such as mandrake, jimsonweed, and tomato. Hyoscyamine is the enantiomer of the well-known nightshade alkaloid atropine.
Both alkaloids are extremely poisonous and have hallucinogenic effects. (Mandrake is sometimes called “insane root”.) They are anticholinergics, and, when used in small doses, they have medical uses such as treating gastrointestinal disorders. Plant extracts containing them have been used medicinally and ritually since biblical times or earlier.
Scopolamine is used criminally to poison people, not only to murder them but also to make them vulnerable to robbery or rape. Despite its adverse effects, it also has been tried as a “truth drug”.
Description
Scopolamine is a type of alkaloid that exists in a variety of Solanaceae plants such
as Scopolia japonica, Datura metel L., and so on. It is the main active ingredient in
these plants.
Apart from scopolamine, several other chemical ingredients also exist in Scopolia
japonica, including hyoscyamine, anisodamine, anisodine, and so on. Hyoscyamine
is an inhibitor of parasympathetic nerve, with the analgesic and antispasmodic functions, especially for sciatica, sometimes for the treatment of epilepsy, seasickness,
etc., and its pharmacological effects are similar to atropine. However, its clinical
application is less because of its toxicity. The clinical applications of anisodamine
are treating infectious toxic shock, vascular disorders, various neuralgia, smooth
muscle spasms, vertigo, fundus disorders and sudden deafness, and other diseases.
It has definite curative effect and is widely used in clinical in China. Its synthetic
product is called “654-2,” which now still is an effective drug to treat infectious
shock and other vascular diseases. While anisodine is used to treat vascular headache, retinal vasospasm, ischemic optic neuritis, cerebrovascular disease, acute
paralysis, central dysfunction caused by carbon monoxide poisoning, tremor, paralysis, bronchial asthma, motion sickness, organophosphorus pesticide poisoning,
and so on .
Chemical properties
White or almost white, crystalline powder or colourless crystals.
Physical properties
Appearance: a kind of viscous liquid, brown color. Solubility: soluble in ethanol, ethyl ether, chloroform, acetone, and water, very soluble in hot water, slightly soluble in benzene and petroleum ether, and also soluble in cold water. It can generate various crystals with multiple inorganic or organic acids. Melting point: 59±1.0?°C
History
Scopolamine is the main active ingredient of Scopolia japonica, Datura metel L.,
and other Solanaceae plants. As early as 1892, the chemist E.?Schmidt first isolated
it from the Scopolia japonica, so named it as scopolamine. Due to its obvious deficiencies in solubility and usage, the researchers modified its structure as scopolamine butylbromide, which was synthesized and used scopolamine and
bromo-n-butane with heating refluxing method. Preparation method of scopolamine
butylbromide is simple, and it’s easy to be synthesized. There are about 170 domestic enterprises producing its raw materials currently.
European Pharmacopoeia (9th ed.), United States Pharmacopeia (36), and the
Japanese Pharmacopoeia (16th ed.) contained bromine scopolamine; British
Pharmacopoeia (2015) contained the raw materials and preparations of scopolamine
butylbromide, including tablets and injections. Pharmacopoeia of the People’s
Republic of China (2015) contained its raw materials, injection, and capsules.
The Uses of Scopolamine
Scopolamine is used for practically the same indications as atropine, but it should be noted that it has a sedative effect on motor activity, and it is recommended for the treatment of Parkinsonian symptoms.
The Uses of Scopolamine
cholinergic (ophthalmic).
Indications
Scopolamine is indicated in adult patients for the prevention of nausea and vomiting associated with motion sickness and for the prevention of postoperative nausea and vomiting (PONV) associated with anesthesia or opiate analgesia.
Background
Scopolamine is a tropane alkaloid isolated from members of the Solanaceae family of plants, similar to atropine and hyoscyamine, all of which structurally mimic the natural neurotransmitter acetylcholine. Scopolamine was first synthesized in 1959, but to date, synthesis remains less efficient than extracting scopolamine from plants. As an acetylcholine analogue, scopolamine can antagonize muscarinic acetylcholine receptors (mAChRs) in the central nervous system and throughout the body, inducing several therapeutic and adverse effects related to alteration of parasympathetic nervous system and cholinergic signalling. Due to its dose-dependent adverse effects, scopolamine was the first drug to be offered commercially as a transdermal delivery system, Scopoderm TTS?, in 1981. As a result of its anticholinergic effects, scopolamine is being investigated for diverse therapeutic applications; currently, it is approved for the prevention of nausea and vomiting associated with motion sickness and surgical procedures.
Scopolamine was first approved by the FDA on December 31, 1979, and is currently available as both oral tablets and a transdermal delivery system.
Definition
ChEBI: A tropane alkaloid that is the (S)-tropic acid ester of 6beta,7beta-epoxy-1alphaH,5alphaH-tropan-3alpha-ol.
brand name
Isopto Hyoscine (Alcon); Transderm-Scop (Ciba-Geigy);Diban;Donnagel-pg;Donnatal;Phenacon;Ru-tuss;Scopoderm tts;Spasmofen;Susano;Tropax.
World Health Organization (WHO)
Scopolamine, an alkaloid with anticholinergic activity extracted from solanaceous plants, was introduced into medicine in 1888. It is used as a mydriatic, as an anti-emetic for the control of motion sickness, and for premedication in general anaesthesia. Shortly after their introduction in the early 1980's, transdermal delivery systems containing scopolamine that were indicated for the prevention of motion sickness were associated with visual disorders (e.g. mydriasis, glaucoma) and hallucinations. The action taken in Norway is in accordance with the legislation in several other countries where these preparations have always been subjected to prescription control.
General Description
Scopolamine (hyoscine) is found in variousmembers of the Solanaceae (e.g., H. niger, Duboisia myoporoides,Scopolia spp., and Datura metel). Scopolamineusually is isolated from the mother liquor remaining from theisolation of hyoscyamine.
Hyoscine is the older name for this alkaloid, althoughscopolamine is the accepted name in the United States.Scopolamine is the levo component of the racemic mixturethat is known as atroscine. The alkaloid is racemized readilyin the presence of dilute alkali.
The alkaloid occurs in the form of a levorotatory, viscousliquid that is only slightly soluble in water but very solublein alcohol, chloroform, or ether. It forms crystalline saltswith most acids, with the hydrobromide being the most stableand the most popularly accepted. An aqueous solution ofthe hydrobromide containing 10% mannitol is said to be lessprone to decomposition than unprotected solutions.
Pharmacokinetics
Scopolamine is an anticholinergic belladonna alkaloid that, through competitive inhibition of muscarinic receptors, affects parasympathetic nervous system function and acts on smooth muscles that respond to acetylcholine but lack cholinergic innervation. Formulated as a patch, scopolamine is released continuously over three days and remains detectable in urine over a period of 108 hours. Scopolamine is contraindicated in angle-closure glaucoma and should be used with caution in patients with open-angle glaucoma due to scopolamine's ability to increase intraocular pressure. Also, scopolamine exhibits several neuropsychiatric effects: exacerbated psychosis, seizures, seizure-like, and other psychiatric reactions, and cognitive impairment; scopolamine may impair the ability of patients to operate machinery or motor vehicles, play underwater sports, or perform any other potentially hazardous activity. Women with severe preeclampsia should avoid scopolamine. Patients with gastrointestinal or urinary disorders should be monitored frequently for impairments, and scopolamine should be discontinued if these develop. Scopolamine can cause blurred vision if applied directly to the eye, and the transdermal patch should be removed before an MRI procedure to avoid skin burns. Due to its gastrointestinal effects, scopolamine can interfere with gastric secretion testing and should be discontinued at least 10 days before performing the test. Finally, scopolamine may induce dependence and resulting withdrawal symptoms, such as nausea, dizziness, vomiting, gastrointestinal disturbances, sweating, headaches, bradycardia, hypotension, and various neuropsychiatric manifestations following treatment discontinuation; severe symptoms may require medical attention.
Pharmacology
Scopolamine butylbromide, a modified scopolamine, is a peripheral anti-choline
drug; it not only can smooth muscle spasm but also block the ganglion and neuromuscular junction and has the weak effect on center. This drug has stronger effects
on intestinal smooth muscle spasm than atropine and anisodamine; it can selectively
relieve gastrointestinal tract, biliary tract, and urinary tract smooth muscle spasm
and inhibit its peristalsis. Scopolamine butylbromide has less effects on the heart,
pupil, and salivary gland, so it rarely induces central nervous excitement, dilation,
inhibition of saliva secretion, and other adverse reactions, which is unlike atropine.
Scopolamine butylbromide is an antagonist of the M cholinergic receptor, its
pharmacological effects are similar to atropine, but it has its own characteristics that
have an obvious advantage in improving blood microcirculation.
Oral absorption of scopolamine butylbromide is not easy. It produces efficacy at
2–4?min after intravenous injection, 8–10?min after subcutaneous or intramuscular
injection, and 20–30? min after oral administration, which could maintain about
2–6?h .
Clinical Use
The commercially available transdermal system of scopolaminecomprises an outer layer of polymer film and a drug reservoircontaining scopolamine, polyisobutylene, and mineral oil, which is interfaced with a microporous membrane tocontrol diffusion of the drug. In this dosage form, scopolamineis effective in preventing motion sickness. The actionis believed to be on the cortex or the vestibular apparatus.Whereas atropine stimulates the CNS, causing restlessnessand talkativeness, scopolamine usually acts as a CNSdepressant.
Safety Profile
Poison by intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by ingestion. Human systemic effects from very small amounts by subcutaneous and intramuscular routes: changes in surface EEG, dstorted perceptions, excitement, hallucinations, and mydriasis. It can cause the individual who is affected to lose a certain amount of his normal inhibitory control. It is for that reason that it has been called “truth serum.” An experimental teratogen. Experimental reproductive effects. Human mutation data reported. In many cases of poisoning from ths material, and even to a certain extent following its medcal application, there is retention of the urine caused by paralysis of the bladder, and catheterization is necessary. The fatal dose is variable. Death has occurred from as little as 0.6 mg, whde recovery has occurred from doses of 7-1 5 mg. An anticholinergc drug. When heated to decomposition it emits highly toxic fumes of NOx. See also ESTERS
Synthesis
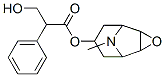
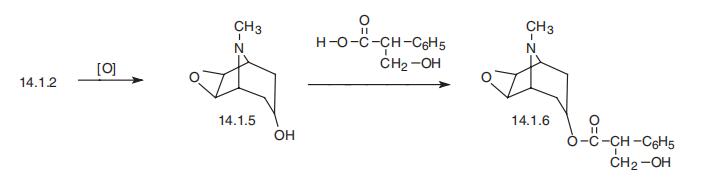
Scopolamine, the L-9-methyl-3-oxa-9-azatricyclo[3.2.1.0.2,4]non-7-yl ester of |á-hydroxymethylphenylacetic acid (14.1.6), can be synthesized from tropenol (14.1.2) by oxidizing the double bond between carbon atoms C6 and C7 of the tropine ring, giving an epoxide derivative, scopine (14.1.5). Esterification of this product using tropic acid gives scopolamine (14.1.6) [7,8].
Metabolism
Little is known about the metabolism of scopolamine in humans, although many metabolites have been detected in animal studies. In general, scopolamine is primarily metabolized in the liver, and the primary metabolites are various glucuronide and sulphide conjugates. Although the enzymes responsible for scopolamine metabolism are unknown, in vitro studies have demonstrated oxidative demethylation linked to CYP3A subfamily activity, and scopolamine pharmacokinetics were significantly altered by coadministration with grapefruit juice, suggesting that CYP3A4 is responsible for at least some of the oxidative demethylation.
Metabolism
It is almost completely metabolized in the liver and is excreted via the kidneys. Its elimination half-life is approximately 8 hours.
Purification Methods
Crystallise it from *benzene/pet ether, EtOH or H2O. It is polymorphic with m 165-166o and 190-191o. The racemate has m 56-57o (H2O), 37-38o (2H2O), syrup (anhydrous), l and d isomers can separate as syrups when anhydrous. [Beilstein 27 H 99, 102, 27 I 247-248, 27 II 43-44, 27 III/IV 1790.]
Properties of Scopolamine
| Melting point: | 59 ºC |
| Boiling point: | 444.28°C (rough estimate) |
| alpha | D20 -28° (c = 2.7) |
| Density | 1.31 |
| refractive index | 1.5022 (estimate) |
| Flash point: | 232.2℃ |
| storage temp. | -20°C |
| solubility | Soluble in water, freely soluble in ethanol (96 per cent). |
| form | neat |
| pka | 7.55-7.81(at 25℃) |
| form | <55°C solid,>55°C liquid |
| color | White to off-white |
| Water Solubility | 95g/L(15 ºC) |
| NIST Chemistry Reference | Scopolamine(51-34-3) |
| EPA Substance Registry System | Benzeneacetic acid, .alpha.-(hydroxymethyl)-, (1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl ester, (.alpha.S)- (51-34-3) |
Safety information for Scopolamine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Scopolamine
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 51-34-3 HYOSCINE EP IMPURITY A 99.90View Details
51-34-3 HYOSCINE EP IMPURITY A 99.90View Details
51-34-3 -
 51-34-3 98%View Details
51-34-3 98%View Details
51-34-3 -
 Scopolamine 98%View Details
Scopolamine 98%View Details
51-34-3 -
 Scopolamine 51-34-3 99%View Details
Scopolamine 51-34-3 99%View Details
51-34-3 -
 Scopolamine 97% CAS 51-34-3View Details
Scopolamine 97% CAS 51-34-3View Details
51-34-3 -
 Hyoscine CAS 51-34-3View Details
Hyoscine CAS 51-34-3View Details
51-34-3 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
