Sasapyrine
Synonym(s):Salsalate;salicylsalicylic acid;2-(2-Hydroxybenzoyl)oxybenzoic acid;2-Hydroxybenzoic acid 2-carboxyphenyl ester;disalicylic acid
- CAS NO.:552-94-3
- Empirical Formula: C14H10O5
- Molecular Weight: 258.23
- MDL number: MFCD00020252
- EINECS: 209-027-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
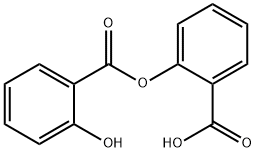
What is Sasapyrine?
Absorption
Salsalate is insoluble in acid gastric fluids (< 0.1 mg/ml at pH 1.0), but readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. A significant portion of the parent compound is absorbed unchanged. The amount of salicylic acid available from salsalate is about 15% less than from aspirin, when the two drugs are administered on a salicylic acid molar equivalent basis (3.6 g salsalate/5 g aspirin). Food slows the absorption of all salicylates including salsalate.
Toxicity
Death has followed ingestion of 10 to 30 g of salicylates in adults, but much larger amounts have been ingested without fatal outcome.
Description
Salsalate, or salicylsalicylic acid, (pKa 3.5 [COOH], 9.8 [AR-OH]) is a dimer of salicylic acid. It is insoluble in gastric juice but is soluble in the small intestine, where it is partially hydrolyzed to two molecules of salicylic acid and absorbed. On a molar basis, it produces 15% less salicylic acid than aspirin. It does not cause GI blood loss and can be given to aspirin-sensitive patients. Salsalate is available as capsules and tablets.
Chemical properties
white crystalline powder
The Uses of Sasapyrine
Nonacetylated (e.g., sodium salicylate) and acetylated (aspirin) forms of salicylate are widely used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain. Sasapyrine is a dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact.
The Uses of Sasapyrine
Nonacetylated aspirin analog. Analgesic, anti-inflammatory.
The Uses of Sasapyrine
Salicylsalicylic acid is used in organic synthesis and functions as a plant hormone, as a key ingredient in topical anti-acne products.
Background
Salsalate is a nonsteroidal anti-inflammatory agent for oral administration. Salsalate's mode of action as an anti-inflammatory and antirheumatic agent may be due to inhibition of synthesis and release of prostaglandins. The usefulness of salicylic acid, the active in vivo product of salsalate, in the treatment of arthritic disorders has been established. In contrast to aspirin, salsalate causes no greater fecal gastrointestinal blood loss than placebo. Salsalate is readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. A significant portion of the parent compound is absorbed unchanged and undergoes rapid esterase hydrolysis in the body. The parent compound has an elimination half-life of about 1 hour. Salicylic acid (the active metabolite) biotransformation is saturated at anti-inflammatory doses of salsalate. Such capacity limited biotransformation results in an increase in the half-life of salicylic acid from 3.5 to 16 or more hours.
Indications
For relief of the signs and symptoms of rheumatoid arthritis, osteoarthritis and related rheumatic disorders.
What are the applications of Application
salicylsalicylic acid is an inhibitor of IKK β and an activator of AMPK.
Definition
ChEBI: A dimeric benzoate ester obtained by intermolecular condensation between the carboxy of one molecule of salicylic acid with the phenol group of a second. It is a prodrug for salycylic acid that is used for treatment of rheumatoid arthritis and osteoarthrit s and also shows activity against type II diabetes.
brand name
Disalcid (3M Pharmaceuticals).
General Description
Salsalate, salicylsalicylic acid (Amigesic, Disalcid,Salflex), is the ester formed between two salicylic acidmolecules. It is rapidly hydrolyzed to salicylic acid followingits absorption. It reportedly causes less gastric irritationthan aspirin, because it is relatively insoluble inthe stomach and is not absorbed until it reaches the smallintestine.
Biochem/physiol Actions
Salsalate is a nonsteroidal anti-inflammatory drug (NSAID), a nonacetylated salicylate with no more problems of gastrointestinal bleeding than placebo. It inhibits synthesis and release of prostaglandins through the inactivation of cyclooxygenase-1 (COX-1) and COX-2. Salsalate is currently being investigated as a treatment for Type 2 diabetes with possible use to prevent the disease in people at risk. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes.
Pharmacokinetics
Salsalate is a nonsteroidal anti-inflammatory agent for oral administration. Salsalate's mode of action as an anti-inflammatory and antirheumatic agent may be due to inhibition of synthesis and release of prostaglandins. The usefulness of salicylic acid, the active in vivo product of salsalate, in the treatment of arthritic disorders has been established. In contrast to aspirin, salsalate causes no greater fecal gastrointestinal blood loss than placebo.
Metabolism
Salsalate is readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. A significant portion of the parent compound is absorbed unchanged and undergoes rapid esterase hydrolysis in the body.
Properties of Sasapyrine
| Melting point: | 139-151 °C |
| Boiling point: | 361.48°C (rough estimate) |
| Density | 1.3326 (rough estimate) |
| refractive index | 1.4825 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥15mg/mL |
| form | Crystalline Powder |
| pka | pKa 3.5 (Uncertain);9.8 (Uncertain) |
| color | white to tan |
| Water Solubility | HYDROLYSIS |
| Sensitive | Moisture Sensitive |
| Merck | 14,8339 |
| CAS DataBase Reference | 552-94-3 |
| NIST Chemistry Reference | 2-Carboxyphenyl salicylate(552-94-3) |
| EPA Substance Registry System | Benzoic acid, 2-hydroxy-, 2-carboxyphenyl ester (552-94-3) |
Safety information for Sasapyrine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Sasapyrine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 552-94-3 Acetylsalicylic Acid EP Impurity E (Salsalate/ Salicylsalicylic acid) 98%View Details
552-94-3 Acetylsalicylic Acid EP Impurity E (Salsalate/ Salicylsalicylic acid) 98%View Details
552-94-3 -
 552-94-3 99%View Details
552-94-3 99%View Details
552-94-3 -
 Salicyl Salicylic Acid 99%View Details
Salicyl Salicylic Acid 99%View Details
552-94-3 -
 552-94-3 Salsalate 98%View Details
552-94-3 Salsalate 98%View Details
552-94-3 -
 552-94-3 98%View Details
552-94-3 98%View Details
552-94-3 -
 Salicylsalicylic acid 95% CAS 552-94-3View Details
Salicylsalicylic acid 95% CAS 552-94-3View Details
552-94-3 -
 Salsalate >98% (HPLC) CAS 552-94-3View Details
Salsalate >98% (HPLC) CAS 552-94-3View Details
552-94-3 -
 Aspirin Impurity E CAS 552-94-3View Details
Aspirin Impurity E CAS 552-94-3View Details
552-94-3
