SAMARIUM
Synonym(s):Samarium;
- CAS NO.:7440-19-9
- Empirical Formula: Sm
- Molecular Weight: 150.36
- MDL number: MFCD00011233
- EINECS: 231-128-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
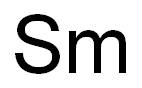
What is SAMARIUM?
Chemical properties
silvery grey powder
Physical properties
Samarium is a hard, brittle, silver-white metal. When freshly cut, it does not tarnish significantlyunder normal room temperature conditions. Four of its isotopes are radioactive andemit alpha particles (helium nuclei). They are Sm-146, Sm-147, Sm-148, and Sm-149.
Its melting point is 1,074°C, its boiling point is 1,794°C, and its density is 7.52g.cm3.
Isotopes
There are 41 known isotopes of samarium. Seven of these are consideredstable. Sm-144 makes up just 3.07% of the natural occurring samarium, Sm-150 makesup 7.38% of natural samarium found on Earth, Sm-152 constitutes 26.75%, and Sm-154 accounts for 22.75%. All the remaining isotopes are radioactive and have very longhalf-lives; therefore, they are considered “stable.” All three contribute to the natural occurrenceof samarium: Sm-147 = 14.99%, Sm-148 = 11.24%, and Sm-149 = 13.82%.
Samarium is one of the few elements with several stable isotopes that occur naturallyon Earth.
Origin of Name
It is named after the mineral samarskite.
Occurrence
Samarium is the 39th most abundant element in the Earth’s crust and the fifth in abundance(6.5 ppm) of all the rare-earths. In 1879 samarium was first identified in the mineralsamarskite [(Y, Ce U, Fe)3 (Nb, Ta, Ti5)O16]. Today, it is mostly produced by the ion-exchangeprocess from monazite sand. Monazite sand contains almost all the rare-earths, 2.8% of whichis samarium. It is also found in the minerals gadolinite, cerite, and samarskite in South Africa,South America, Australia, and the southeastern United States. It can be recovered as a byproductof the fission process in nuclear reactors.
History
Discovered spectroscopically by its sharp absorption lines in 1879 by Lecoq de Boisbaudran in the mineral samarskite, named in honor of a Russian mine official, Col. Samarski. Samarium is found along with other members of the rare-earth-elements in many minerals, including monazite and bastnasite, which are commercial sources. The largest producer of rare-earth minerals is now China, followed by the U.S., India, and Russia. It occurs in monazite to the extent of 2.8%. While misch metal containing about 1% of samarium metal has long been used, samarium has not been isolated in relatively pure form until recently. Ion-exchange and solvent extraction techniques have recently simplified separation of the rare earths from one another; more recently, electrochemical deposition, using an electrolytic solution of lithium citrate and a mercury electrode, is said to be a simple, fast, and highly specific way to separate the rare earths. Samarium metal can be produced by reducing the oxide with barium or lanthanum. Samarium has a bright silver luster and is reasonably stable in air. Three crystal modifications of the metal exist, with transformations at 734 and 922°C. The metal ignites in air at about 150°C. Thirty-three isotopes and isomers of samarium are now recognized. Natural samarium is a mixture of seven isotopes, three of which are unstable but have long half-lives. Samarium, along with other rare earths, is used for carbonarc lighting for the motion picture industry. The sulfide has excellent high-temperature stability and good thermoelectric efficiencies up to 1100°C. SmCo5 has been used in making a new permanent magnet material with the highest resistance to demagnetization of any known material. It is said to have an intrinsic coercive force as high as 2200 kA/m. Samarium oxide has been used in optical glass to absorb the infrared.Samarium is used to dope calcium fluoride crystals for use in optical masers or lasers. Compounds of the metal act as sensitizers for phosphors excited in the infrared; the oxide exhibits catalytic properties in the dehydration and dehydrogenation of ethyl alcohol. It is used in infrared absorbing glass and as a neutron absorber in nuclear reactors. The metal is priced at about $3.50/g (99.9%). Little is known of the toxicity of samarium; therefore, it should be handled carefully.
Characteristics
Samarium is somewhat resistant to oxidation in air but will form a yellow oxide over time. Itignites at the rather low temperature of 150°C. It is an excellent reducing agent, releases hydrogenwhen immersed in water, and has the capacity to absorb neutrons in nuclear reactors.
The Uses of SAMARIUM
Samarium is easy to magnetize, but very difficult to demagnetize. This makes it ideal forthe manufacture of permanent magnets (SmCo5) that are part of the hard disks for computers.Samarium is also used as a neutron absorber in nuclear reactors, as well as for lasers andmetallurgical research. It makes up about 1% of the metals in misch metal, an alloy in cigarettelighter flints. It is also one of several rare-earths used in floodlights and carbon-arc lights usedby the motion picture industry. Samarium is used as a catalyst in several industries, includingthe dehydrogenation of ethanol alcohol.
The Uses of SAMARIUM
Arylsulfonyl chlorides and arylsulfinates are reduced by a combination of samarium and titanium(IV) chloride to make diaryl disulfides. It is a neutron absorber, dopant for laser crystals.
Definition
A silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Samarium is used in the metallurgical, glass, and nuclear industries. Symbol: Sm; m.p. 1077°C; b.p. 1791°C; r.d. 7.52 (20°C); p.n. 62; r.a.m. 150.36.
Definition
samarium: Symbol Sm. A soft silverymetallic element belonging tothe lanthanoids; a.n. 62; r.a.m.150.35; r.d. 7.52 (20°C); m.p. 1077°C;b.p. 1791°C. It occurs in monaziteand bastnatite. There are seven naturallyoccurring isotopes, all of whichare stable except samarium–147,which is weakly radioactive (half-life2.5 × 1011 years). The metal is usedin special alloys for making nuclearreactorparts as it is a neutron absorber.Samarium oxide (Sm2O3) isused in small quantities in special opticalglasses. The largest use of the elementis in the ferromagnetic alloySmCo5, which produces permanentmagnets five times stronger than anyother material. The element was discoveredby Fran?ois Lecoq de Boisbaudranin 1879.
Hazard
The salts of samarium are toxic if ingested. These salts react with water, liberating hydrogen,which may explode.
Properties of SAMARIUM
| Melting point: | 1074 °C (lit.) |
| Boiling point: | 1794 °C (lit.) |
| Density | 7.47 g/mL at 25 °C (lit.) |
| storage temp. | Store below +30°C. |
| form | powder |
| color | Silvery-gray |
| Specific Gravity | 7.4 |
| Resistivity | 91.4 μΩ-cm, 0°C |
| Water Solubility | Insoluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,8425 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-19-9(CAS DataBase Reference) |
| EPA Substance Registry System | Samarium (7440-19-9) |
Safety information for SAMARIUM
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for SAMARIUM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

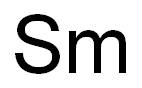
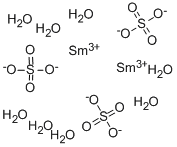
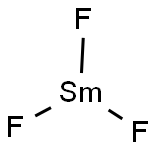
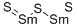
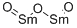
You may like
-
 Samarium, ingot CAS 7440-19-9View Details
Samarium, ingot CAS 7440-19-9View Details
7440-19-9 -
 Samarium foil, 0.127mm (0.005 in.) thick CAS 7440-19-9View Details
Samarium foil, 0.127mm (0.005 in.) thick CAS 7440-19-9View Details
7440-19-9 -
 Samarium foil, 0.127mm (0.005 in.) thick CAS 7440-19-9View Details
Samarium foil, 0.127mm (0.005 in.) thick CAS 7440-19-9View Details
7440-19-9 -
 Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
7440-19-9 -
 Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
7440-19-9 -
 Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
Samarium rod, 6.35mm (0.25 in.) dia. CAS 7440-19-9View Details
7440-19-9 -
 Samarium CAS 7440-19-9View Details
Samarium CAS 7440-19-9View Details
7440-19-9 -
 Samarium rod, 12.7mm (0.5 in.) dia. CAS 7440-19-9View Details
Samarium rod, 12.7mm (0.5 in.) dia. CAS 7440-19-9View Details
7440-19-9