S3I-201
Synonym(s):2-Hydroxy-4-(((4-methylphenyl)sulfonyloxy)acetyl)amino)-benzoic acid, NSC 74859;2-Hydroxy-4-[[[[(4-methylphenyl)sulfonyl]oxy]acetyl]amino]-benzoic acid;NSC 74859;STAT3 Inhibitor VI, S3I-201 - CAS 501919-59-1 - Calbiochem
- CAS NO.:501919-59-1
- Empirical Formula: C16H15NO7S
- Molecular Weight: 365.36
- MDL number: MFCD09907564
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
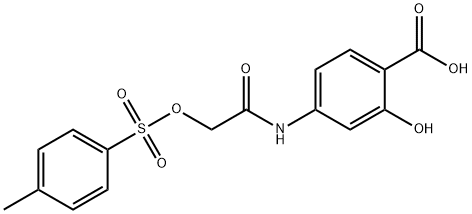
What is S3I-201?
The Uses of S3I-201
S3I-201 has been used as a signal transducer and activator of transcription 3 (STAT3) inhibitor:
- to confirm the role of STAT3 phosphorylation in interleukin (IL)-33 production in lung epithelial cells and IL-22 mRNA expression in sorted group 3 innate lymphoid cells (ILC3s)
- to study the cellular response of STAT3 triggered by β-hexaclorocyclohexane (β-HCH) in various cell lines
- to examine the influence of STAT3 in response to angiotensin II (ang II) on induction of fibrotic proteins in kidney epithelial cells
The Uses of S3I-201
A chemical probe inhibitor of STAT3 with an IC50 of 86 μM.
The Uses of S3I-201
S2I-201 is a small molecule inhibitor that provides a pathway to rational combination therapies for Melanoma.
What are the applications of Application
Stat3 Inhibitor VI, S3I-201 is an inhibitor of Stat3 phosphorylation and dimerization
Definition
ChEBI: An amidobenzoic acid obtained by formal condensation of the carboxy group of [(4-methylbenzene-1-sulfonyl)oxy]acetic acid with the amino group of 4-amino-2-hydroxybenzoic acid.
Biological Activity
s3i-201 is a selective inhibitor of stat3 with ic50 value of 86 μm [1].in the in vitro stat3 dna-binding assay, s3i-201 showed potent inhibition of the stat3 dna-binding activity with an average ic50 of 86 μm. in the emsa assay, s3i-201 selectively inhibited stat3 dna-binding activity over that of stat1 and stat5. it suppressed the complex formation of stat1-stat3 and stat1-stat1 with ic50 values of 160 and > 300 μm, respectively. besides that, the unphosphorylated, inactive stat3 monomer was found to restore the stat3 dna-binding activity inhibited by s3i-201, suggesting that the inhibition was independent on the activation status. in nih 3t3/v-src fibroblasts, s3i-201 inhibited the constitutive activation of stat3 and reduced the ptyr-705 stat3 levels. moreover, s3i-201 was found to significantly induce apoptosis in cells with constitutively active stat3 at concentration of 30–100 μm. s3i-201 also reduced the expression of cyclin d1, bcl-xl and surviving in these cells [1].
Biochem/physiol Actions
S3I-201 is a cell-permeable Stat3 inhibitor that binds to the Stat3-SH2 domain, prevents Stat3 phosphorylation/activation, dimerization, and DNA-binding.
storage
-20°C
References
[1] siddiquee k, zhang s, guida w c, et al. selective chemical probe inhibitor of stat3, identified through structure-based virtual screening, induces antitumor activity. proceedings of the national academy of sciences, 2007, 104(18): 7391-7396.
Properties of S3I-201
| Melting point: | >185oC (dec.) |
| Boiling point: | 654.7±55.0 °C(Predicted) |
| Density | 1.507 |
| storage temp. | -20°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 2.98±0.10(Predicted) |
| color | white to beige |
Safety information for S3I-201
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for S3I-201
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

![5-[(4-chlorophenyl)methylidene]-1,3-thiazolidine-2,4-dione](https://img.chemicalbook.in/CAS/GIF/24138-83-8.gif)
You may like
-
 STAT3 Inhibitor VI, S3I-201 CASView Details
STAT3 Inhibitor VI, S3I-201 CASView Details -
 STAT3 Inhibitor VI, S3I-201 CAS 501919-59-1View Details
STAT3 Inhibitor VI, S3I-201 CAS 501919-59-1View Details
501919-59-1 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
