S-(-)-Carbidopa
Synonym(s):(S)-3-(3,4-Dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid;S-(−)-α-Hydrazino-3,4-dihydroxy-2-methylbenzenepropanoic acid
- CAS NO.:28860-95-9
- Empirical Formula: C10H14N2O4
- Molecular Weight: 226.23
- MDL number: MFCD00069231
- EINECS: 657-445-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
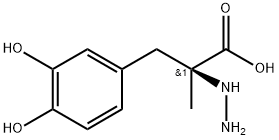
What is S-(-)-Carbidopa?
Absorption
When levodopa/carbidopa is administered orally, 40-70% of the administered dose is absorbed. Once absorbed, carbidopa shows bioavailability of 58%. A maximum concentration of 0.085 mcg/ml was achieved after 143 min with an AUC of 19.28 mcg.min/ml.
Toxicity
The LD50 of carbidopa is reported to be in the rat of 4810 mg/kg. In animal studies, carbidopa showed no incidences on neoplasia and showed no effect on the fertility status and development.
No reports of overdosage have been registered with the carbidopa-only product. In the event of overdosage, immediate gastric lavage is recommended as well as intravenous fluid administration. Continuous electrocardiographic monitoring is required.
Chemical properties
White Solid
Originator
Sinemet,Merck Sharp and Dohme,Italy,1974
The Uses of S-(-)-Carbidopa
in combinaison with levodopa as antiparkinsonian;inhibits aromatic-L-amino-acid decarboxylase (DOPA Decarboxylase or DDC)
The Uses of S-(-)-Carbidopa
(S)-(-)-Carbidopa is a peripheral decarboxylase inhibitor that is commonly used in combination with L-DOPA (D533751) for treatment of Parkinsonism. S(-)-Carbidopa ( has also been shown to prolong the elimination half-life of L-DOPA from blood plasma and skeletal muscle.
Indications
Carbidopa is indicated with levodopa for the treatment of symptoms of idiopathic Parkinson disease, postencephalitic parkinsonism and symptomatic parkinsonism followed by carbon monoxide or manganese intoxication.
The combination therapy is administered for the reduction of levodopa-driven nausea and vomiting.
The product of carbidopa should be used in patients where the combination therapy of carbidopa/levodopa provide less than the adequate daily dosage.
As well carbidopa can be used in patients where the dosages of carbidopa and levodopa require individual titration.
Background
Carbidopa presents a chemical denomination of N-amino-alpha-methyl-3-hydroxy-L-tyrosine monohydrate. It potently inhibits aromatic amino acid decarboxylase (DDC) and due to its chemical properties, it does not cross the blood-brain barrier. Due to its activity, carbidopa is always administered concomitantly with levodopa. An individual formulation containing solely carbidopa was generated to treat nausea in patients where the combination therapy levodopa/carbidopa is not efficient reducing nausea.
The first approved product by the FDA containing only carbidopa was developed by Amerigens Pharmaceuticals Ltd and approved on 2014. On the other hand, the combination treatment of carbidopa/levodopa was originally developed by Watson Labs but the historical information by the FDA brings back to the approval of this combination therapy developed by Mayne Pharma in 1992.
What are the applications of Application
S(?)-Carbidopa is a DDC inhibitor
Definition
ChEBI: 3-(3,4-Dihydroxyphenyl)propanoic acid in which the hydrogens alpha- to the carboxyl group are substituted by hydrazinyl and methyl groups (S-configuration). Carbidopa is a dopa decarboxylase inhibitor, so prevents conversi n of levodopa to dopamine. It has no antiparkinson activity by itself, but is used (commonly as its hydrate) in the management of Parkinson's disease to reduce peripheral adverse effects of levodopa.
Manufacturing Process
To a solution of vanillin in toluene is added nitroethane, butylamine and glacial
acetic acid. The mixture is refluxed and the water of reaction is steadily
azeotropically removed by distillation. After the theoretical amount of water is
distilled out, distillation is continued to remove excess reactants. The last
trace of excess reactants is then removed at room temperature under a
vacuum. The product is then triturated with a hydrocarbon solvent such as
Skellysolve B and is thus obtained in a crystalline state. In general, however,
it is preferred to dissolve the residue directly in toluene for use in the next
step, without isolating the 1-(2-nitropropen-1-yl)-4-hydroxy-3-
methoxybenzene.
A mixture of iron, ferric chloride and water is added to the toluene solution.
The mixture is heated to reflux and concentrated hydrochloric acid is added
dropwise at a rate calculated to keep the mixture refluxing vigorously. After
the hydrochloric acid is all added, the refluxing is continued by the application
of heat for several hours. A siliceous filter aid is then added to the cooled
reaction mixture and the material is removed by filtration. The filter cake is
washed four times, each time with 90 ml of benzene. The organic layer is then
separated from the filtrate. The water layer is acidified to a pH of 2 and
extracted three times with 90 ml portions of benzene.
These extracts are then combined with the organic solvent layer and the
combined organic phase is extracted four times with 100 ml portions of water.
It is then stirred for an hour with 230 ml of 10% sodium bisulfite solution.
The organic solvent phase is then separated, washed seven times with 100 ml
portions of water and dried over magnesium sulfate. Evaporation of the
solvent gives 1-(4-hydroxy-3-methoxyphenyl)-2-propanone in the form of an
oil.
A mixture of 59.5 g of that oily product, 1.85 liters of benzene and 1 kg of
potassium bisulfite in 200 liters of water is stirred at room temperature for
two hours. The precipitated bisulfite addition product of the ketone is isolated
by filtration and washed with isopropanol and then with ether. Five hundred
grams of the adduct is mixed with 119.5 g of potassium cyanide, 292 ml of
85% hydrazine hydrate and 910 ml of water. The mixture is stirred overnight
at room temperature after which the product is isolated by filtration. The
product is washed 3 times with 250 ml portions of water and then 3 times
with 230 ml portions of ether. It is then air dried and vacuum dried at room
temperature.
Fifty cubic centimeters of concentrated hydrochloric acid is saturated with
hydrogen chloride gas at -10°C. To the solution is then added 2.5 g of the
intermediate product, of the formula shown above, slowly with vigorous
stirring. The mixture is allowed to stir overnight while warming at room
temperature gradually. It is then concentrated in vacuo to a syrup. To the
residual syrup is added 100 ml of 48% hydrobromic acid. The reaction vessel
is purged with nitrogen and the reaction mixture is then refluxed for 3 hours
after which it is concentrated in vacuo to a mixture of a syrup and a solid. The residue is taken up in sufficient water to form a clear solution. Activated
charcoal is added and the mixture is heated to boiling and filtered.
The filtrate is concentrated to dryness in vacuo and the residue is taken up in
25 cc of ethanol. The residual ammonium bromide is removed by filtration and
to the filtrate there is added sufficient diethylamine to change the pH to 6.4.
The mixture is warmed to 60°C and then cooled to room temperature. It is
then allowed to stand overnight to effect complete crystallization. It is then
cooled to 0°C and the product is isolated by filtration, washed with methanol
and air dried. The product (α-hydrazino-α-methyl-β-(3,4-dihydroxyphenyl)-
propionic acid) is recrystallized once from water using a proportion of 15 cc
water per gram of product.
brand name
Lodosyn (Bristol-Myers Squibb).
Therapeutic Function
Muscle relaxant, Antiparkinsonian
General Description
Carbidopa is used for treating restless legs (RL) syndrome and periodic leg movements in sleep (PLMS). Carbidopa along with levodopa is used to treat Parkinson′s disease.
Biological Activity
Peripheral decarboxylase inhibitor, used in combination with levodopa for treatment of Parkinsonism.
Biochem/physiol Actions
Peripheral inhibitor of L-aromatic amino acid decarboxylase. When co-administered with L-DOPA, it prevents extra-CNS conversion to dopamine, thereby increasing CNS concentrations of effective compounds. Selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells by increasing H2O2 in these cell lines, which are sensitive to reactive oxygen species.
Pharmacokinetics
When mixed with levodopa, carbidopa inhibits the peripheral conversion of levodopa to dopamine and the decarboxylation of oxitriptan to serotonin by aromatic L-amino acid decarboxylase. This results in an increased amount of levodopa and oxitriptan available for transport to the central nervous system. Carbidopa also inhibits the metabolism of levodopa in the GI tract, thus, increasing the bioavailability of levodopa.
The presence of additional units of circulating levodopa can increase the effectiveness of the still functional dopaminergic neurons and it has been shown to alleviate symptoms for a time. The action of carbidopa is very important as levodopa is able to cross the blood-brain barrier while dopamine cannot. Hence the administration of carbidopa is essential to prevent the transformation of external levodopa to dopamine before reaching the main action site in the brain.
The coadministration of carbidopa with levodopa has been shown to increase the half-life of levodopa more than 1.5 times while increasing the plasma level and decreasing clearance. The combination therapy has also shown an increase of the recovery of levodopa in urine instead of dopamine which proves a reduced metabolism. This effect has been highly observed by a significant reduction in levodopa requirements and a significant reduction in the presence of side effects such as nausea. It has been observed that the effect of carbidopa is not dose-dependent.
Metabolism
The loss of the hydrazine functional group (probably as molecular nitrogen) represents the major metabolic pathway for carbidopa. There are several metabolites of carbidopa metabolism including 3-(3,4-dihydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methylpropionic acid, 3-(3-hydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methyllactic acid, 3-(3-hydroxyphenyl)-2-methyllactic acid, and 3,4-dihydroxyphenylacetone (1,2).
storage
Store at RT
Properties of S-(-)-Carbidopa
| Melting point: | 203-205° (dec); mp 208° |
| Boiling point: | 367.84°C (rough estimate) |
| alpha | D -17.3° (methanol) |
| Density | 1.2616 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 3.40±0.14(Predicted) |
| form | powder |
| color | White to Off-White |
| Stability: | Unstable in Solution |
| CAS DataBase Reference | 28860-95-9(CAS DataBase Reference) |
Safety information for S-(-)-Carbidopa
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for S-(-)-Carbidopa
S-(-)-Carbidopa manufacturer
Divis Laboratories Ltd
Ralington Pharma
Vijaya Pharma And Life Science
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
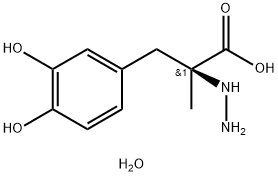


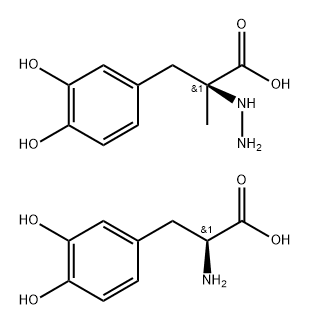

You may like
-
 28860-95-9 Carbidopa 99%View Details
28860-95-9 Carbidopa 99%View Details
28860-95-9 -
 28860-95-9 98%View Details
28860-95-9 98%View Details
28860-95-9 -
 Carbidopa 98%View Details
Carbidopa 98%View Details
28860-95-9 -
 Carbidopa 28860-95-9 98%View Details
Carbidopa 28860-95-9 98%View Details
28860-95-9 -
 Carbidopa 98%View Details
Carbidopa 98%View Details
28860-95-9 -
 Carbidopa 98%View Details
Carbidopa 98%View Details
28860-95-9 -
 Carbidopa >95% CAS 28860-95-9View Details
Carbidopa >95% CAS 28860-95-9View Details
28860-95-9 -
 S-(−)-Carbidopa CAS 28860-95-9View Details
S-(−)-Carbidopa CAS 28860-95-9View Details
28860-95-9