28860-95-9
Product Name:
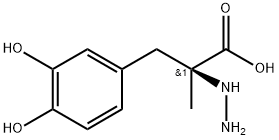
S-(-)-Carbidopa
Formula:
C10H14N2O4
Synonyms:
(S)-3-(3,4-Dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid;S-(−)-α-Hydrazino-3,4-dihydroxy-2-methylbenzenepropanoic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Boiling Point | Decomposes |
| Melting Point | 203-208 °C |
| Solubility | 3.8 mg/L |
| LogP | -1.9 |
| Dissociation Constants | 2.3 |
| Collision Cross Section | 142.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 226.23 g/mol |
|---|---|
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 226.09535693 g/mol |
| Monoisotopic Mass | 226.09535693 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 261 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Carbidopa (anhydrous) is 3-(3,4-Dihydroxyphenyl)propanoic acid in which the hydrogens alpha- to the carboxyl group are substituted by hydrazinyl and methyl groups (S-configuration). Carbidopa is a dopa decarboxylase inhibitor, so prevents conversion of levodopa to dopamine. It has no antiparkinson activity by itself, but is used (commonly as its hydrate) in the management of Parkinson's disease to reduce peripheral adverse effects of levodopa. It has a role as an EC 4.1.1.28 (aromatic-L-amino-acid decarboxylase) inhibitor, an antiparkinson drug and a dopaminergic agent. It is a member of hydrazines, a monocarboxylic acid and a member of catechols.
