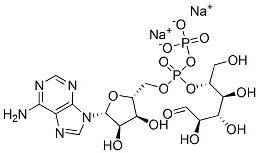
S-Adenosyl-L-methionine
- CAS NO.:29908-03-0
- Empirical Formula: C15H22N6O5S
- Molecular Weight: 398.44
- MDL number: MFCD00871208
- EINECS: 249-946-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-30 18:52:02
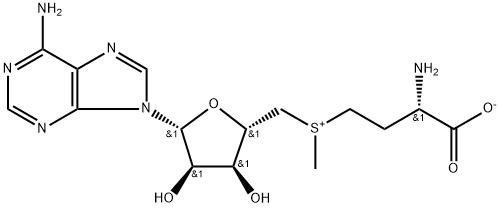
What is S-Adenosyl-L-methionine?
Absorption
S-Adenosylmethionine is absorbed from the small intestine following oral intake. As absorption is affected by food, it is best to take on an empty stomach. Bioavailability is low following oral intake.
Toxicity
Irritating to mucus membranes and upper respiratory tract. Can cause CNS depression.
Originator
Donamet ,Ravizza
Occurrence
SAM-e is found in all living cells and is a precursor in some amino acids.
The Uses of S-Adenosyl-L-methionine
white powder LOD 0.2%
Indications
S-Adenosylmethionine (SAMe) is used as a drug in Europe for the treatment of depression, liver disorders, fibromyalgia, and osteoarthritis. It has also been introduced into the United States market as a dietary supplement for the support of bone and joint health, as well as mood and emotional well being.
Background
Physiologic methyl radical donor involved in enzymatic transmethylation reactions and present in all living organisms. It possesses anti-inflammatory activity and has been used in treatment of chronic liver disease. (From Merck, 11th ed)
What are the applications of Application
Ademetionine is a common co-substrate involved in methyl group transfers to nucleic acids, proteins and lipids.
Definition
ChEBI: S-adenosyl-L-methioninate is a sulfonium betaine that is a conjugate base of S-adenosyl-L-methionine obtained by the deprotonation of the carboxy group. It has a role as a human metabolite. It is functionally related to a L-methioninate. It is a conjugate base of a S-adenosyl-L-methionine.
Manufacturing Process
S-Adenosyl methionine (SAM) is produced is prepared by cultivating of
Saccharomyces cerevisiae.
One loopful of each of the microorganism strains (IFO 2342, IFO 2343, IFO
2345, IFO 2346, IFO 2347) was inoculated in 10 ml of a heat-sterilized culture
medium adjusted to pH 6.0 and composed of 5.0 g/dl of glucose, 0.5 g/dl of
polypeptone, 0.4 g/dl of KH2PO4, 0.4 g/dl of K2HPO4, 0.02 g/dl of
MgSO4·7H2O and 0.2 g/dl of yeast extract, and cultivated with shaking at
28°C for 24 h.
1 L of a culture medium adjusted to pH 6.0 and composed of 10.0 g/dl of
sucrose, 1.0 g/dl of yeast extract, 0.4 g/dl of K2HPO4, 0.01 g/dl of
MgSO4·7H2O, 1.5 g/dl of urea (separately sterilized), 0.75 g/dl of Lmethionine,
0.02 g/dl of CaCl2·2H2O, 0.25 mg/dl of ZnSO4·7H2O, 0.25 mg/dl
of FeSO4·7H2O, 125.0 mg/dl of MnSO4·6H2O, 2.0 μg/dl of CuSO4·5H2O, 2.0
μg/dl of H3BO3, 0.2 μg/dl of CoCl2·6H2O and 1.0 μg/dl of KI was put in a 2-
liter fermentor and sterilized. Then, 5 ml of the seed culture broth prepared
as above was inoculated in the culture medium and cultivated at 28°C for 72
h with aeration and agitation.
After the cultivation, the microbial cells were collected by centrifugal
separation, washed once with physiological saline, suspended in 100 ml of 1.5
N perchloric acid, and shaken at room temperature for 1 h. The suspension
was then centrifuged to remove the microbial cells, and the resulting liquid
was adjusted to pH 4.5 by adding potassium hydrogen carbonate. The
resulting precipitate of potassium perchlorate was removed by centrifugal
separation to give an extract containing SAM. The amount of SAM in the
extract was determined, and the amount of SAM based on the dry cells.
The extract in an amount of 0.2 g as SAM was passed through a column filled
with 50 ml of Amberlite IRC-50 (H+ form), a weakly acidic cation exchange
resin, to cause adsorption of SAM. 0.005 N acetic acid was passed through the
column to wash it until the absorbance at 260 nm of the eluate becames less
than 0.1. Thus, impurities were removed. Then, 0.1 N sulfuric acid was
passed through the column, and SAM was eluted until the absorbance at 260
nm of the eluate becames less than 0.05. The eluate was treated with
Amberlite IRA 900 resin (OH- form) to adjust its pH to 3.0, and then
lyophilized to obtain SAM sulfate. The SAM based may be produced from SAM
sulfate by treatment with potassium hydrogen carbonate. The purity of SAM
was measured by cellulose thin-layer chromatography, paper chromatography
and high-performance liquid chromatography. The yield of SAM based on the
dry cells: IFO 2343-12.1%; IFO 2346-18.8%; IFO 2347-16.7%.
Therapeutic Function
Metabolic, Antiinflammatory
Benefits
S-Adenosyl-L-methionine (SAMe) plays a role in the immune system, maintains cell membranes, and helps produce and break down brain chemicals like serotonin, melatonin, and dopamine. It works with vitamin B12 and folate (vitamin B9). Being deficient in either vitamin B12 or folate may reduce SAMe levels in your body. Several studies show that SAMe helps relieve the pain of osteoarthritis. Taking SAMe by mouth works, as well as ibuprofen and other similar drugs for reducing symptoms of osteoarthritis. Other studies suggest that SAMe may help treat depression.
Pharmacokinetics
S-adenosylmethionine is an intermediate metabolite of methionine. Its involvement in methylation assists in cellular growth and repair, maintains the phospho-bilipid layer in cell membranes. It also helps in the maintenance of the action of several hormones and neurotransmitters that affect mood. Highest concentration are found in the brain and the liver.
Metabolism
Significant first-pass metabolism in the liver. Approximately 50% of S-Adenosylmethionine (SAMe) is metabolized in the liver. SAMe is metabolized to S-adenosylhomocysteine, which is then metabolized to homocysteine. Homocysteine can either be metabolized to cystathionine and then cysteine or to methionine. The cofactor in the metabolism of homocysteine to cysteine is vitamin B6. Cofactors for the metabolism of homocysteine to methionine are folic acid, vitamin B12 and betaine.
Properties of S-Adenosyl-L-methionine
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Sonicated), Water (Slightly) |
| form | Solid |
| color | White to Yellow |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 29908-03-0(CAS DataBase Reference) |
Safety information for S-Adenosyl-L-methionine
Computed Descriptors for S-Adenosyl-L-methionine
| InChIKey | MEFKEPWMEQBLKI-WGIZDKKNNA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](C[S+](C)CC[C@H](N)C(=O)[O-])O[C@H]1N1C=NC2C(=NC=NC1=2)N)O |&1:1,2,3,9,15,r| |
S-Adenosyl-L-methionine manufacturer
Meteoric Biopharmaceuticals Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
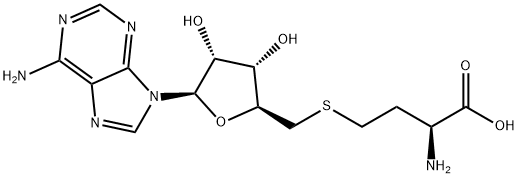
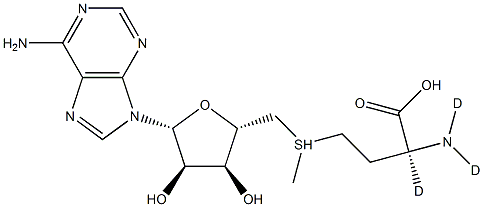
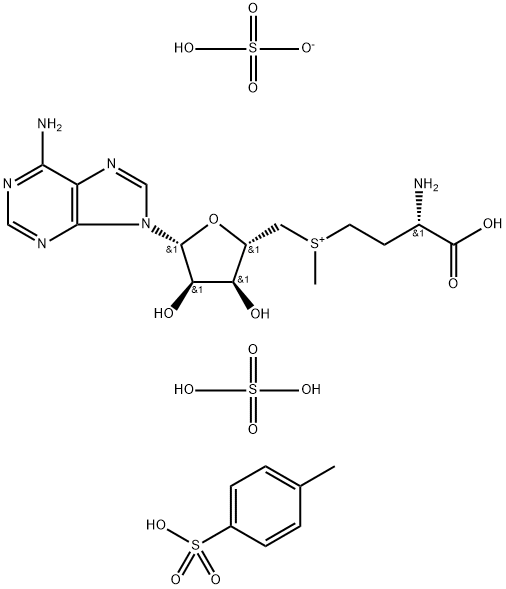



You may like
-
 29908-03-0 S-(5'-Adenosyl)L-methionine 98%View Details
29908-03-0 S-(5'-Adenosyl)L-methionine 98%View Details
29908-03-0 -
 S-Adenosyl-L-methionine, 99% CAS 29908-03-0View Details
S-Adenosyl-L-methionine, 99% CAS 29908-03-0View Details
29908-03-0 -
 Ademetionine 97% CAS 29908-03-0View Details
Ademetionine 97% CAS 29908-03-0View Details
29908-03-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1