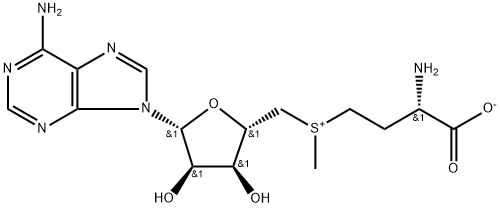
5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
Synonym(s):5′-Deoxy-S-adenosyl-L -homocysteine;AdoHcy;S-(5′-Adenosyl)-L -homocysteine;S-(5′-Deoxyadenosine-5′)-L -homocysteine
- CAS NO.:979-92-0
- Empirical Formula: C14H20N6O5S
- Molecular Weight: 384.41
- MDL number: MFCD00037388
- EINECS: 213-560-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:00
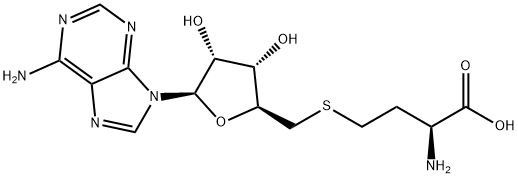
What is 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE?
Description
S-
Chemical properties
Pale Beige Solid
The Uses of 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
A novel biomarker for predicting susceptibility of a subject to a mental or neurodegenerative disorder.
The Uses of 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
SAH is suitable for use:
- to investigate whether AdoHcy competes with AdoMet in the down-regulation of reporter activity of LUC reporter gene
- as a reagent to study the abundance patterns of SAH and its correlation with vertebrate metamorphosis
- in the optimization of the protein (lysine K) methyltransferase SET7/9 activity assay
- in the fluorescence polarization (FP) assay during dengue virus methyltransferase activity measurement
- as a standard for the measurement of SAH from blood samples by high performance liquid chromatography (HPLC) with fluorimetric detection method
What are the applications of Application
S-(5′-Adenosyl)-L-homocysteine is an amino acid derivative that is an important intermediate in metabolic pathways
Definition
ChEBI: The S-adenosyl derivative of L-homocysteine.
Biochem/physiol Actions
S-(5′-Adenosyl)-L-homocysteine (AdoHcy/SAH) is a component of intracellular homocysteine stress. AdoHcy is a competitive inhibitor (versus AdoMet) of DNA methyltransferases (S-adenosyl-L-methionine (AdoMet)-dependent methyltransferases) involved in epigenetics. Consequently, AdoHcy is used in a variety of studies on epigenetics in hyperhomocysteinemic states. AdoHcy is metabolized by S-adenosylhomocysteine hydrolase (AHCY). AdoHcy is the product of enzymatic transmethylation reactions involving S-Adenosylmethionine (SAM). It is reconverted to SAM by its cleavage into adenosine and L-homocysteine, a substrate of thetin-homocysteine S-methyltransferase. The concentration alterations of SAM and SAH in plasma serve as predictors of cellular methylation potential and metabolic alterations. Methylation capacity indicates specific genetic polymorphisms and/or nutritional deficiencies. Methylation is important in epigenetics, reprogramming, and cancer.
Purification Methods
It has been recrystallised several times from aqueous EtOH or H2O to give small prisms and has max 260nm in H2O. The picrate has m 170o(dec) from H2O. [Baddiley & Jameison J Chem Soc 1085 1955, de la Haba & Cantoni J Biol Chem 234 603 1959, Borchardt et al. J Org Chem 41 565 1976, NMR: Follmann et al. Eur J Biochem 47 187 1974, Beilstein 26 III/IV 3676.]
Properties of 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
| Melting point: | 209-211 °C |
| Boiling point: | 787.5±70.0 °C(Predicted) |
| Density | 1.91±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | 1 M HCl: soluble19.60 - 20.40mg/mL, clear to slightly hazy, colorless to faintly yellow |
| pka | 2.22±0.10(Predicted) |
| form | crystalline |
| color | Off-White to Brown |
| BRN | 99188 |
| Stability: | Hygroscopic |
Safety information for 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
Computed Descriptors for 5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE
| InChIKey | ZJUKTBDSGOFHSH-WFMPWKQPSA-N |
5'-DEOXY-S-ADENOSYL-L-HOMOCYSTEINE manufacturer
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![ADENOSYL-L-METHIONINE, S-[CARBOXYL-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB7362189.gif)
You may like
-
 S-(5-Adenosyl)-L-Homocysteine extrapure CAS 979-92-0View Details
S-(5-Adenosyl)-L-Homocysteine extrapure CAS 979-92-0View Details
979-92-0 -
 S-Adenosyl-L-homocysteine CAS 979-92-0View Details
S-Adenosyl-L-homocysteine CAS 979-92-0View Details
979-92-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1