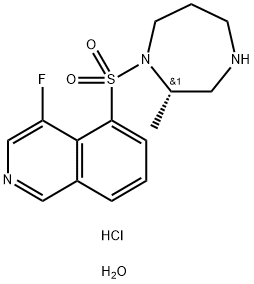
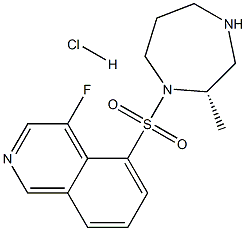
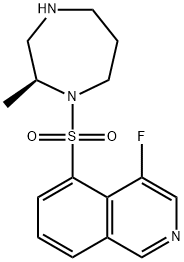
Ripasudil hydrochloride dihydrate
- CAS NO.:887375-67-9
- Empirical Formula: C15H21ClFN3O3S
- Molecular Weight: 377.86
- MDL number: MFCD26960897
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:11
What is Ripasudil hydrochloride dihydrate?
Description
Ripasudil hydrochloride hydrate (Glanatec®) was approved in Japan in 2014 for the treatment of glaucoma and ocular hypertension. Originally discovered by D. Western Therapeutics Institute, Inc. and licensed by the Kowa Company, Ltd, ripasudil functions as a selective Rho-kinase inhibitor and reduces intraocular pressure by stimulation of aqueous humour drainage of the trabecular meshwork. While this recent approval allows for use of ripasudil as a twice-daily monotherapy treatment when other drugs cannot be used or are not effective, clinical trials using ripasudil as a combination therapy with other glaucoma drugs have shown promising results in the treatment of primary open-angle glaucoma or ocular hypertension. Currently, the Kowa Company is also pursuing trials focused on the use of ripasudil for the treatment of diabetic retinopathy and diabetic macular edema.
The Uses of Ripasudil hydrochloride dihydrate
Ripasudil (Glanatec TM, Kowa Pharmaceutical), a close derivative of fasudil, is another Rho kinase inhibitor approved in Japan at the end of 2014 for the treatment of glaucoma and ocular hypertensionwhen other therapeutic agents are not effective or cannot be administered. Additionally, ripasudil has been tested in diabetic retinopathy clinical trials and shown to promote corneal endothelial cell proliferation, endothelium regeneration, and wound healing.
The Uses of Ripasudil hydrochloride dihydrate
Ripasudil Hydrochloride Dihydrate is a derivative of fasudil and a rho kinase inhibitor (1,2). It is used for the treatment of glaucoma and ocular hypertension.
Synthesis
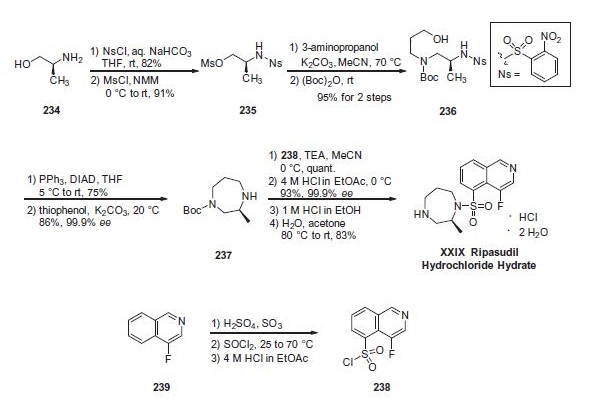
While initial synthetic routes to ripasudil were carried out via a stepwise functionalization of 4-fluoroisoquinoline-5-sulfonyl chloride (238), more recent reports describe an efficient route to ripasudil employing a late stage-coupling of Boc-diazepane (237) with 4-fluoroisoquinoline-5-sulfonyl chloride (238), enabling synthesis on multi-kilogram scale and isolation of the drug in high purity. This optimized route to ripasudil begins with 2-nitrobenzene sulfonyl chloride (NsCl)- mediated protection of (S)-2-amino-1-propanol (234) in 82% yield. In this case, use of the NaHCO3/THF/H2O conditions were essential for preventing bis-nosylation.228 Alcohol activation with methanesulfonyl chloride (MsCl) in N-methyl morpholine (NMM) took place smoothly to give the corresponding mesylate 235 in 91% yield. Direct mesylate displacement with 3-aminopropanol and subsequent amine protection as the carbamate ((Boc)2O) in a one-pot fashion provided the corresponding Boc-amino propanol product 236 in 95% yield over 2 steps. With the acyclic diazepane precursor 236 in hand, employment of the intramolecular Fukuyama-Mitsunobu N-alkyl cyclization conditions (diisopropyl azodicarboxylate (DIAD)/PPh3) allowed generation of the diazepane in 75% yield. Nosyl group cleavage with thiophenol/K2CO3 provided the Boc-diazepane 237 in 65% overall yield and 98% purity following a pH-controlled aqueous workup. Finally, 4-fluoroisoquinoline- 5-sulfonyl chloride (238)?aprepared via subjection of 4- fluoroisoquinoline (239) to sulfur trioxide and sulfuric acid followed by treatment with thionyl chloride and finally 4 N HCl in ethyl acetate?awas involved in a 1-pot, two-step procedure in which this sulfonyl chloride was coupled with diazepane 237 (TEA/MeCN) to access the ripasudil framework in quantitative yield. Synthesis of the final drug target by deprotection with 4 M HCl in ethyl acetate followed by neutralization with aqueous sodium hydroxide provided the free base of ripasudil in 93% yield and 99.8% purity. Conversion to the more stable hydrochloride dihydrate form could be performed by treatment of the free base with 1 M HCl/EtOH and subsequent heating of the hydrochloride in H2O/acetone to provide ripasudil hydrochloride dihydrate XXIX in 83% yield.
in vivo
in optic nerve crush (nc) c57bl/6 mice model, oral administration of k-115 (1 mg/kg/d) increased 34 ± 3% survival of rgcs after nc [2].
References
shimokawa h, takeshita a. rho-kinase is an important therapeutic target in cardiovascular medicine[j]. arteriosclerosis, thrombosis, and vascular biology, 2005, 25(9): 1767-1775.yamamoto k, maruyama k, himori n, et al. the novel rho kinase (rock) inhibitor k-115: a new candidate drug for neuroprotective treatment in glaucomanovel rho kinase inhibitor[j]. investigative ophthalmology & visual science, 2014, 55(11): 7126-7136.tanihara h, inoue t, yamamoto t, et al. phase 1
Properties of Ripasudil hydrochloride dihydrate
| storage temp. | Store at -20°C |
| solubility | insoluble in EtOH; ≥123.2 mg/mL in DMSO; ≥41.7 mg/mL in H2O |
| form | solid |
| color | White to off-white |
| Stability: | Hygroscopic |
Safety information for Ripasudil hydrochloride dihydrate
Computed Descriptors for Ripasudil hydrochloride dihydrate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1