Riociguat
- CAS NO.:625115-55-1
- Empirical Formula: C20H19FN8O2
- Molecular Weight: 422.42
- MDL number: MFCD19443708
- EINECS: 641-755-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-10 22:08:48
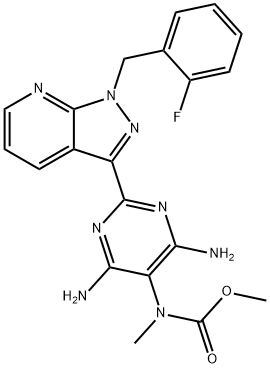
What is Riociguat?
Absorption
The pharmacokinetics of riociguant are dose proportional from 0.5 mg to 2.5 mg. The absolute bioavailability is approximately 94%. After oral administration, peak plasma concentrations were achieved within 1.5 hours. Food does not affect the bioavailability of riociguat.
Toxicity
EMBRYO-FETAL TOXICITY Do not administer Riociguat to a pregnant female because it may cause fetal harm. Females of reproductive potential: Exclude pregnancy before the start of treatment, monthly during treatment, and 1 month after stopping treatment. Prevent pregnancy during treatment and for one month after stopping treatment by using acceptable methods of contraception. For all female patients, Riociguat is available only through a restricted program called the Adempas Risk Evaluation and Mitigation Strategy.
Description
In September 2013, Health Canada approved riociguat (also referred to as BAY 63-2521), for the treatment of patients with chronic thromboembolic pulmonary hypertension (CTEPH) after surgical treatment or inoperable CTEPH and for the treatment of adults with pulmonary arterial hypertension (PAH). Riociguat has a dual mode of action and works by (a) sensitizing sGC to the body’s NO by stabilizing NO–sGC binding and (b) an NO-independent, direct stimulation of sGC via a different binding site. This process restores the NO–sGC–cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation.
Headache, dizziness, dyspepsia/gastritis, nausea, diarrhea, hypotension, vomiting, anemia, gastroesophageal reflux, and constipation were the most common adverse events ( 3%) observed during riociguat clinical trials. Riociguat comes with a black box warning for embryo-fetal toxicity.
Chemical properties
Brownish Orange Solid
Originator
Bayer (Germany)
The Uses of Riociguat
Riociguat is used in the treatment for pulmonary hypertension.
The Uses of Riociguat
Riocguat is used in the treatment for pulmonary hypertension.
Background
Riociguat is a soluble guanylate cyclase (sGC) agonist approved in the USA, Europe and several other regions for patients with group I PAH (pulmonary arterial hypertension) in WHO FC II or III; and for the treatment of patients with inoperable CTEPH (chronic thromboembolic pulmonary hypertension), or persistent/recurrent PH (pulmonary hypertension) after pulmonary endarterectomy in WHO FC II or III. Riociguat is marketed under the brand Adempas? by Bayer HealthCare Pharmaceuticals. Treatment with riociguat costs USD $7,500 for 30 days of treatment.
Indications
Riociguat is indicated for the treatment of adults with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH), (WHO Group 4) after surgical treatment, or inoperable CTEPH, to improve exercise capacity and WHO functional class. Riociguat is indicated for the treatment of adults with pulmonary arterial hypertension (PAH), (WHO Group 1), to improve exercise capacity, WHO functional class and to delay clinical worsening. Efficacy was shown in patients on Riociguat monotherapy or in combination with endothelin receptor antagonists or prostanoids. Studies establishing effectiveness included predominately patients with WHO functional class II–III and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (25%).
Definition
ChEBI: A carbamate ester that is the methyl ester of {4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}methylcarbamic acid. It is used for treatment of chronic thromboembolic pulmonary hypertension an pulmonary arterial hypertension
brand name
Adempas
Clinical Use
Guanylate cyclase stimulator:
Treatment of chronic thromboembolic pulmonary
hypertension (CTEPH) and pulmonary arterial
hypertension (PAH)
Synthesis
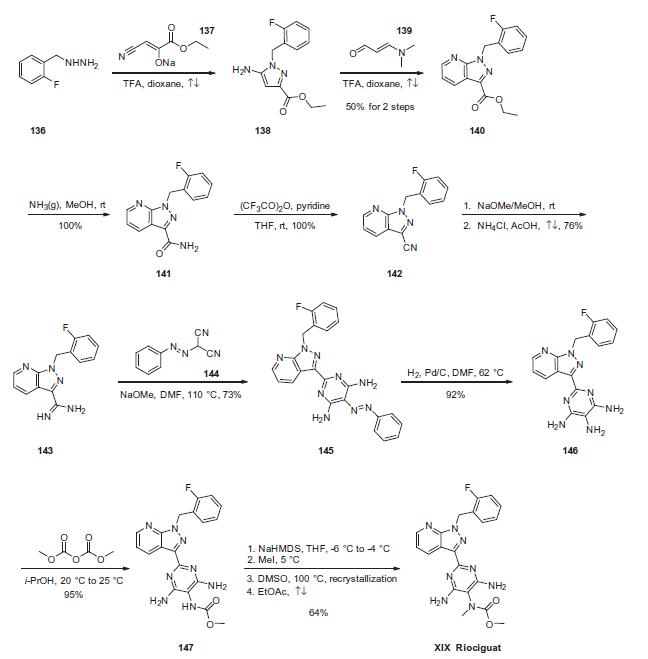
The sequence began with condensation of commercial 2-fluorobenzylhydrazine (136) with sodium ethyl cyanopyruvate (137), which derives from diethyl oxalate to generate aminopyrazole 138. This was followed by the cyclocondensation with 3-dimethylaminoacrolein (139) to access pyrazolopyridine 140 in 50% yield for the two-step operation. Next, ester 140 was transformed to the corresponding primary amide 141, which was subsequently dehydrated upon treatment with trifluoroacetic acid anhydride (TFAA) to construct nitrile 142 in quantitative yield from 140. Subjection of cyanopyrazole 142 to Pinner conditions using methoxide and ammonium chloride in refluxing acetic acid generated amidine 143, and this was followed by condensation with the malononitrile derivative 144 in base to provide pyrimidine 145 in 73% yield. Hydrogenative cleavage of the phenyldiazine converted 145 to the pyrimidyl triamine 146, which underwent carbamoylation at the 40 position to produce the penultimate carbamate 147. This carbamate was then selectively methylated through deprotonation of the carbamate N¨CH proton followed by quench with methyl iodide. Sequential recrystallization from warm DMSO and refluxing ethyl acetate produced riociguat (XIX) in 64% yield from 147.
Drug interactions
Potentially hazardous interactions with other drugs
Avanafil, sildenafil, tadalafil, vardenafil: enhanced
hypotensive effect - avoid.
Nicorandil: possibly enhanced hypotensive effect -
avoid.
Nitrates: possibly enhanced hypotensive effect -
avoid.
Metabolism
The active metabolite (M1) of riociguat is 1/3 to 1/10 as potent as riociguat.
Metabolism
N-demethylation, catalysed by CYP1A1, CYP3A4,
CYP2C8 and CYP2J2 is the major biotransformation
pathway leading to its major circulating active
metabolite M-1 (pharmacological activity: 1/10th to
1/3rd of riociguat) which is further metabolised to the
pharmacologically inactive N-glucuronide.
Riociguat and metabolites are excreted via both
renal (33-45%) and biliary/faecal routes (48-59%).
Approximately 9-44% of the administered dose was
found as unchanged riociguat in faeces.
Properties of Riociguat
| Melting point: | 247-251°C (dec.) |
| Boiling point: | 567.2±50.0 °C(Predicted) |
| Density | 1.51 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 1.51±0.50(Predicted) |
| color | Off-White to Orange |
Safety information for Riociguat
Computed Descriptors for Riociguat
Riociguat manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![Methyl [4,6-diaMino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyriMidin-5-yl]carbaMate](https://img.chemicalbook.in/CAS/20150408/GIF/625115-52-8.gif)

![2-[1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyriMidine-4,5,6-triaMine](https://img.chemicalbook.in/CAS/20150408/GIF/428854-24-4.gif)
![4,6-PyriMidinediaMine, 2-[1-[(2-fluorophenyl)Methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-(phenylazo)-](https://img.chemicalbook.in/CAS/20150408/GIF/428854-23-3.gif)
You may like
-
 625115-55-1 Riociguat 98%View Details
625115-55-1 Riociguat 98%View Details
625115-55-1 -
 625115-55-1 98%View Details
625115-55-1 98%View Details
625115-55-1 -
 Riociguat 98% (HPLC) CAS 625115-55-1View Details
Riociguat 98% (HPLC) CAS 625115-55-1View Details
625115-55-1 -
 Riociguat 97% CAS 625115-55-1View Details
Riociguat 97% CAS 625115-55-1View Details
625115-55-1 -
 Riociguat 625115-55-1 98%View Details
Riociguat 625115-55-1 98%View Details
625115-55-1 -
 625115-55-1 Riociguat 98%View Details
625115-55-1 Riociguat 98%View Details
625115-55-1 -
 Riociguat for FP system suitability CAS 625115-55-1View Details
Riociguat for FP system suitability CAS 625115-55-1View Details
625115-55-1 -
 Riociguat CAS 625115-55-1View Details
Riociguat CAS 625115-55-1View Details
625115-55-1
