Ramiprilat
- CAS NO.:87269-97-4
- Empirical Formula: C21H28N2O5
- Molecular Weight: 388.46
- MDL number: MFCD00865787
- EINECS: 258-696-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
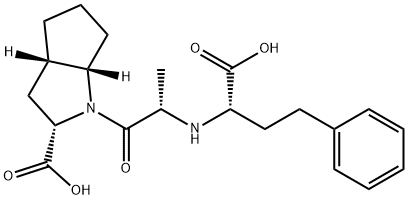
What is Ramiprilat?
Description
Ramiprilat is the active metabolite of ramipril and functions as an angiotensin-converting enzyme inhibitor (pKi = 9.08 in human heart) to suppress the conversion of angiotensin I to angiotensin II and the degradation of bradykinin, thereby preventing vasoconstriction. Furthermore, ramiprilat is reported to interfere with the targeting of B2 kinin receptors to endothelial cell membranes further preventing bradykinin signaling. In addition to its cardioprotective effects in both animal models and clinical studies, ramiprilat has been reported to inhibit matrix metalloproteinase-3 and -9 activity in isolated Crohn’s disease fistulas.
Chemical properties
White to Off-White Solid
The Uses of Ramiprilat
Ramiprilat (Ramipril EP Impurity E) is a metabolite of Ramipril (R111000), and acts as an ACE inhibitor (1). Reduces the need for dialysis among patients previously on angiotensin-converting-enzyme inhibitors (2). Ramipril is used in stroke prevention. (3)
The Uses of Ramiprilat
A metabolite of Ramipril (R111000).
What are the applications of Application
Ramiprilat is the active metabolite of Ramipril that functions as an inhibits angiotensin-converting enzymes.
Definition
ChEBI: A dipeptide that is the active metabolite of ramipril. An angiotensin-converting enzyme (ACE) inhibitor, used to treat high blood pressure and congestive heart failure.
in vitro
pretreatment with ramiprilat could significantly attenuate the recovery of b2 kinin receptors in while increasing that from membranes lacking caveolin, and such effect was not because of the inhibition of bradykinin degradation. ramiprilat could also decrease [3h]bradykinin binding to cr membranes. in addition, ramiprilat treatment led to reactivation of the b2 receptor in bradykinin-stimulated cells [1].
in vivo
in previous animal study, when compared with the control rats, diabetic rats showed decreased creatinine clearance rate, increased urinary protein excretion and blood pressure, as well as development of tubulointerstitial fibrosis, glomerulosclerosis, and inflammatory cell infiltration. furthermore, the blocking angiotensin ii with ramipril (the prodrug of ramiprilat) was able to significantly improve all of these parameters [2].
References
[1] t. benzing, i. fleming, a. blaukat, et al. angiotensin-converting enzyme inhibitor ramiprilat interferes with the sequestration of the b2 kinin receptor within the plasma membrane of native endothelial cells. circulation 99(15), 2034-2040 (1999).
[2] li c, yang cw, park cw, ahn hj, kim wy, yoon kh, suh sh, lim sw, cha jh, kim ys, kim j, chang ys, bang bk. long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. kidney int. 2003 feb;63(2):454-63.
[3] warner gt, perry cm. ramipril: a review of its use in the prevention of cardiovascular outcomes. drugs. 2002;62(9):1381-405.
Properties of Ramiprilat
| Melting point: | 139-141°C |
| Boiling point: | 632.2±55.0 °C(Predicted) |
| Density | 1.273±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 2.20±0.10(Predicted) |
| color | White to Pale Yellow |
| Stability: | Hygroscopic |
Safety information for Ramiprilat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ramiprilat
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
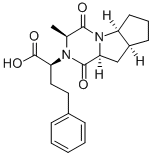
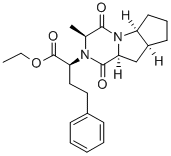
![Ramipril Related Compound A (30 mg) ((2S,3aS,6aS)-1-[(S)2-[[(S)-1-(methoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-octahydrocyclopenta[b]pyrrole-2-carboxylic acid)](https://img.chemicalbook.in/CAS/20180808/GIF/108313-11-7.gif)
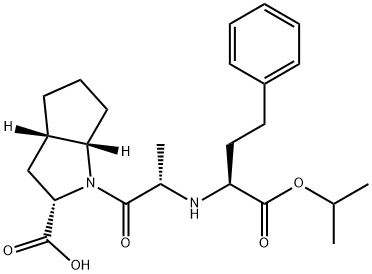

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1