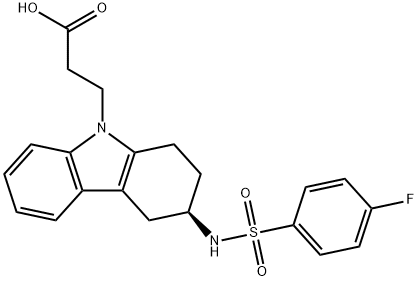
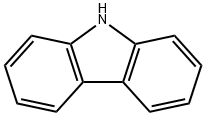
Ramatroban
Synonym(s):(+)-(3R)-3-[[(4-Fluorophenyl)sulfonyl]amino]-1,2,3,4-tetrahydro-9H-carbazole-9-propanoic acid;BAY u3405
- CAS NO.:116649-85-5
- Empirical Formula: C21H21FN2O4S
- Molecular Weight: 416.47
- MDL number: MFCD00887606
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Ramatroban?
Description
Ramatroban, originally developed for the treatment of cardiovascular pathologies as thromboembolism, was finally introduced in Japan for the treatment of allergic rhinitis. It is a (3R)-enantiomer that can be synthesized in 5 steps from 3-oxo-1,2,3,4- tetrahydrocarbazole by stereoselective reductive amination, using S-phenethylamine as the chiral source, followed by hydrogenolysis, sulfonylation and finally 2-step Ncarboxyethylation. Ramatroban is a potent antagonist of prostaglandin receptors (PGD2, PGH2) and thromboxane receptors (TxA2); accordingly, it blocks the contractions induced by thromboxane or TxA2-mimetics in animal and human airway smooth muscle. It also prevents, when administered i.v., p.o. or by aerosol, bronchoconstriction induced by PGD2 or antigen. In animal models of nasal allergy, ramatroban inhibits antigen-induced neutrophil infiltration into nasal mucosa and also inhibits nasal symptoms. In several species including humans, it is well-absorbed, extensively protein-bound (>95%) and eliminated mainly as a glucuro-conjugate; in man, its terminal half-life is 2 to 3 hours.
Chemical properties
WHite to Off-White Solid
Originator
Bayer (Germany)
The Uses of Ramatroban
A thromboxane receptor antagonist for use in the treatment of coronary artery disease.
What are the applications of Application
BAY-u 3405 is a suppresor PGD2-induced migration of human eosinophils
Definition
ChEBI: Ramatroban is an organic molecular entity.
brand name
Baynas
Biological Activity
Potent dual antagonist of TP/DP 2 (CRTH2) prostanoid receptors (K i values are 4.3, 4.5 and > 10000 nM for hDP 2 , hTP and hDP 1 receptors respectively). Suppresses PGD 2 -induced migration of human eosinophils (IC 50 = 170 nM).
in vitro
bay u3405 showed significant inhibitory effects on the binding of 3h-labeled pgd2 to crth2, albeit with much lower potency. bay u3405 and indomethacin also inhibited pgd2-induced ca2+ mobilization in crth2 transfectants to almost the same extent. however, indomethacin but not bay u3405 was confirmed as an agonist of ca2+ mobilization at concentrations greater than 10 nm [1].
in vivo
for rat with splanchnic artery occlusion shock, administration of bay u3405 at 30 mg/kg i.v. could significantly increase the survival time and survival rate, improve mean arterial blood pressure, reduce the plasma levels of myocardial depressant factor, partially restore macrophage phagocytosis and lower mpo activity in both the ileum and the lung [2].
storage
Desiccate at -20°C
References
[1] sugimoto, h. ,shichijo, m.,iino, t., et al. an orally bioavailable small molecule antagonist of crth2, ramatroban (bay u3405), inhibits prostaglandin d2-induced eosinophil migration in vitro. journal of pharmacology and experimental therapeutics 305, 347-352 (2003).
[2] canale p, squadrito f, altavilla d, ioculano m, campo gm, squadrito g, urna g, sardella a, caputi ap. beneficial effects of bay u3405, a novel thromboxane a2 receptor antagonist, in splanchnic artery occlusion shock. pharmacology. 1994 dec;49(6):376-85.
[3] aizawa h, shigyo m, nogami h, hirose t, hara n. bay u3405, a thromboxane a2 antagonist, reduces bronchial hyperresponsiveness in asthmatics. chest. 1996 feb;109(2):338-42.
Properties of Ramatroban
| Melting point: | 134-135° |
| Boiling point: | 654.7±65.0 °C(Predicted) |
| alpha | D +70.1° (c = 1.0 in methanol) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: ≥40mg/mL |
| form | solid |
| pka | 4.60±0.10(Predicted) |
| color | white |
| CAS DataBase Reference | 116649-85-5(CAS DataBase Reference) |
Safety information for Ramatroban
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ramatroban
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Ramatroban >95% CAS 116649-85-5View Details
Ramatroban >95% CAS 116649-85-5View Details
116649-85-5 -
 Ramatroban CAS 116649-85-5View Details
Ramatroban CAS 116649-85-5View Details
116649-85-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1