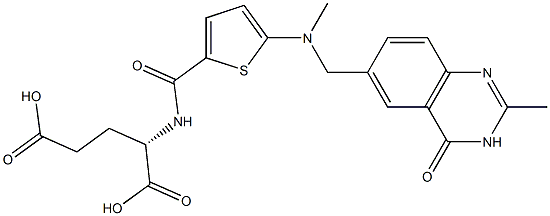
Raltitrexed
Synonym(s):L -Glutamic acid, N-((5-(((1,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl)methylamino)-2-thienyl)carbonyl);ICI-D-1694;ZD-1694
- CAS NO.:112887-68-0
- Empirical Formula: C21H22N4O6S
- Molecular Weight: 458.49
- MDL number: MFCD00864168
- EINECS: 652-997-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-19 11:58:51
What is Raltitrexed?
Toxicity
Side effects include pale skin, troubled breathing, unusual bleeding or bruising, unusual tiredness or weakness, black, tarry stools, chest pain, chills, cough, fever, painful or difficult urination, shortness of breath, sore throat, sores, ulcers, or white spots on lips or in mouth, swollen glands, increase in bowel movements, loose stools, soft stools.
Description
Tomudex was launched in Ireland, France, Luxembourg and the UK for advanced colorectal cancer and it was prepared in a convergent manner (6 steps) from diethyl L-glutamate and 6-bromomethyi-2-methyl-quinazolin4(3H)-one. Tomudex is a highly selective inhibitor of thymidylate synthase (TS), the key enzyme in the biochemical conversion of dUMP to dTMP. It enters the cell via the reduced folatefmethotrexate cell membrane carrier and is converted to the polyglutamate species by folylpolyglutamate synthase within 4h where it then binds to the folate substrate site of TS. Clinically, it had a 29% response rate in patients with advanced colorectal cancer. It is water soluble, can be administered as a single dose every three weeks and had no hepto- or nephrotoxicity.
Chemical properties
Yellow Crystalline Powder
Originator
Zeneca (UK)
The Uses of Raltitrexed
Folate-based inhibitor of thymidylate synthase; rapidly and extensively metabolized to its more potent polyglutamate derivatives. Antineoplastic
What are the applications of Application
Raltitrexed is an inhibitor of thymidylate synthase and DHFR
Indications
For the treatment of malignant neoplasm of colon and rectum
Background
Raltitrexed (brand name Tomudex?) is a chemotherapy drug manufactured AstraZeneca Company, is an antimetabolite used in chemotherapy. It is an inhibitor of thymidylate synthase.
Definition
ChEBI: ICI D1694 is a N-acyl-amino acid.
brand name
Tomudex (Zeneca).
Biochem/physiol Actions
Raltitrexed is a folate-based inhibitor of thymidylate synthase (TS) that is rapidly and extensively metabolized to its more potent polyglutamate derivatives. By inhibiting the formation of precursor pyrimidine nucleotides, raltitrexed prevents the formation of DNA and RNA, which are required for the growth and survival of both normal cells and cancer cells.
Pharmacokinetics
Raltitrexed belongs to a group of medicines known as antimetabolites. It is used to treat cancer of the colon and rectum. It may also be used to treat other kinds of cancer, as determined by your doctor. Raltitrexed blocks an enzyme needed by the cell to live. This interferes with the growth of cancer cells, which are eventually destroyed. Since the growth of normal body cells may also be affected by raltitrexed, other effects will also occur. Some of these may be serious and must be reported to your doctor. Other effects, like hair loss, may not be serious but may cause concern.
Clinical Use
Treatment of colorectal cancer when fluorouracil and folinic acid cannot be used
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Folic and folinic acid: impairs cytotoxic action -
avoid.
Metabolism
Raltitrexed is transported into cells via a reduced folate carrier. Inside the cell it is extensively polyglutamated by the enzyme folyl polyglutamate synthetase to polyglutamate forms.
Metabolism
Raltitrexed is actively transported into cells and
metabolised to active polyglutamate forms.
The remainder of a dose is not metabolised and is
excreted unchanged, about 50% of a dose appearing in the
urine, and about 15% in the faeces.
Properties of Raltitrexed
| Melting point: | 176-1800C |
| Density | 1.49±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥10mg/mL |
| form | solid |
| pka | 3.50±0.10(Predicted) |
| color | off-white to light yellow |
| CAS DataBase Reference | 112887-68-0(CAS DataBase Reference) |
Safety information for Raltitrexed
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. |
Computed Descriptors for Raltitrexed
| InChIKey | IVTVGDXNLFLDRM-HNNXBMFYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Raltitrexed CAS 112887-68-0View Details
Raltitrexed CAS 112887-68-0View Details
112887-68-0 -
 Raltitrexed CAS 112887-68-0View Details
Raltitrexed CAS 112887-68-0View Details
112887-68-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
