radium oxide
- CAS NO.:12143-02-1
- Empirical Formula: HORa
- Molecular Weight: 243.01
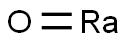
What is radium oxide?
Physical properties
Radium Oxide can be prepared by heating radium
metal in air to give a mixture of white radium oxide,
RaO, and radium nitride, Ra3N2. The superoxide RaO2
is also likely to form in this reaction.
2Ra(solid)+ O2(gas) → 2RaO(solid)
Ra(solid)+ O2(gas) → RaO2(solid)
3Ra(solid)+N2(gas) → Ra3N2(solid)
Radium reacts very readily with water to form
radium hydroxide, Ba(OH)2 and hydrogen gas (H2). The reaction is expected to be more violent than that of
barium:
Ra(solid)+ 2H2O(gas)→ Ra(OH)2(aq)+H2(gas)
The Uses of radium oxide
Radium oxide is often used as a precursor to the formation of other radium compounds which are used in radiotherapy of humans.
Safety information for radium oxide
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

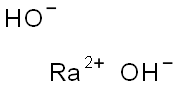
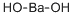

You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
