BERYLLIUM HYDROXIDE
- CAS NO.:13327-32-7
- Empirical Formula: BeH2O2
- Molecular Weight: 43.03
- MDL number: MFCD00049856
- EINECS: 236-368-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
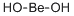
What is BERYLLIUM HYDROXIDE?
Chemical properties
White powder. Decomposes to the oxide at 138C. Soluble in acids and alkalies; insoluble in water.
Physical properties
Beryllium Hydroxide can be prepared by reacting
the oxide with water:
BeO+H2O→Be(OH)2
If heated, it decomposes at
1273°C. Its solubility in water is slight and its solubility
product Ksp(H2O) is 6.92×10-22. It is soluble in acids and
bases.
Beryllium hydroxide exists in three forms: as a metastable
tetragonal crystalline solid (a-Be(OH)2; as a stable
orthorhombic crystalline solid (b-Be(OH)2); and in
a slightly basic pH, it appears as a slimy, gelatinous
substance (Be(OH)42-).
The Uses of BERYLLIUM HYDROXIDE
Beryllium hydroxide is a strategic component
concerning the usage of beryllium metal and its
compounds in industry. Beryllium hydroxide is the key compound used to prepare a variety of beryllium products.Note that Be(OH)2 is the primary product used to prepare the metal and various beryllium salts.
The largest usage of beryllium hydroxide is to make
the oxide or the metal that is used in many industries.
The total tonnage is not large but substantial.
Beryllium hydroxide is available commercially in large quantities.
The Uses of BERYLLIUM HYDROXIDE
manufacture of beryllium and beryllium oxide.
Definition
beryllium hydroxide: A whitecrystalline compound, Be(OH)2, precipitatedfrom solutions of berylliumsalts by adding alkali. Like the oxide,it is amphoteric and dissolves in excessalkali to give beryllates.
Preparation
Beryllium hydroxide, Be(OH)2, is an amphoteric
hydroxide, dissolving in both acids and alkalis. Industrially
it is produced as a by-product in the extraction of
beryllium metal from the ores, “beryl” and “bertrandite”
When alkali is added to beryllium salt solutions, the
a-form (a gel) is formed. If this left to stand or boiled
the rhombic b-form precipitates. This has the Zn(OH)2
structure with tetrahedral beryllium centers. The alkali
dissolution forms a “beryllate”:
2NaOH(aq)+Be(OH)2(solid) → Na2Be(OH)4(aq)
With acids, beryllium salts are formed involving the
Be(OH)+ cation. With sulfuric acid, H2SO4:
2Be(OH)2+H2SO4→[Be(OH)]2SO4+ 2H2O
Thus, the probable formula for beryllium hydroxide
is Be(OH)OH. However, this has never been reported.
Beryllium hydroxide dehydrates completely at 400°C to form a white powder of acid soluble beryllium oxide:
Be(OH)2+ heat → BeO+H2O
Further heating at higher temperature produces acid
insoluble BeO.
Hazard
Very toxic.
Safety Profile
Confirmed carcinogen withexperimental carcinogenic and tumorigenic data. Poisonby intravenous route. When heated to decomposition it emitsvery toxic fumes of BeO.
Properties of BERYLLIUM HYDROXIDE
| Melting point: | 105 °C (decomp) |
| Density | 1.92 |
| solubility | slightly soluble in H2O, alkaline solutions; soluble in acid solutions |
| form | amorphous powder or crystals |
| color | Colorless, orthorhombic, amorphous powder orcrystals |
| PH | 7.9(1 mM solution);7.9(10 mM solution);7.9(100 mM solution) |
| Water Solubility | very slightly soluble H2O, dilute alkali; soluble hot NaOH, acids [MER06] |
| EPA Substance Registry System | Beryllium hydroxide (Be(OH)2) (13327-32-7) |
Safety information for BERYLLIUM HYDROXIDE
Computed Descriptors for BERYLLIUM HYDROXIDE
BERYLLIUM HYDROXIDE manufacturer
A.B.ENTERPRISES
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 13327-32-7 Beryllium hydroxide 98%View Details
13327-32-7 Beryllium hydroxide 98%View Details
13327-32-7 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1