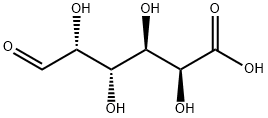
(R)-3-Hydroxybutyric acid
- CAS NO.:625-72-9
- Empirical Formula: C4H8O3
- Molecular Weight: 104.1
- MDL number: MFCD00066257
- EINECS: 210-909-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
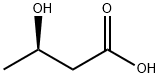
What is (R)-3-Hydroxybutyric acid?
Description
(R)-3-Hydroxybutanoic acid is a metabolite, and converted from acetoacetic acid catalyzed by 3-hydroxybutyrate dehydrogenase. It is a monomer of PHB (poly[(R)-3-hydroxybutyrate]) with wide industrial and medical applications. It can also serve as chiral precursor for synthesis of pure biodegradable PHB and its copolyesters.
The Uses of (R)-3-Hydroxybutyric acid
(R)-3-Hydroxybutyric acid may be used in the preparation of copolymers by reacting with various hydroxyalkanoic acids. It may also be used in the preparation of (3R,4R)-4-acetoxy-3-[(R)-1-(formyloxy)ethyl]-2-azetidinone.
Definition
ChEBI: (R)-3-hydroxybutyric acid is the R-enantiomer of 3-hydroxybutyric acid. Involved in the synthesis and degradation of ketone bodies, it can be used as an energy source by the brain during hypoglycaemia, and for the synthesis of biodegradable plastics. It is a sex pheremone in the European spider Linyphia triangularis. It has a role as a human metabolite, a pheromone and a fungal metabolite. It is a ketone body and a 3-hydroxybutyric acid. It is a conjugate acid of a (R)-3-hydroxybutyrate. It is an enantiomer of a (S)-3-hydroxybutyric acid.
Synthesis
Procedure for the Synthesis of (R)-3-Hydroxybutyric acid:Into a 50 ml flask was added 5N KOH (10 ml) which was cooled to 0 °C. Methyl [R]-3-Hydroxybutyrate (5 g) was then added with stirring over 1.5-h time period, and the temperature was maintained at 0 °C for 24 h. The reaction was terminated by the slow addition of 6N HC1 (8.3 ml) with stirring at 5 °C The resultant aqueous solution was then saturated with solid NaCl and extracted 20 times with 20 mL portions of diethyl ether. The organic extract was dried over anhydrous MgSO4 and the ether removed by rotary evaporation. The product was a white crystalline solid (3.8 g, yield 87%). NMR and IR were used to characterize the product.
storage
Room temperature
Properties of (R)-3-Hydroxybutyric acid
| Melting point: | 49-50 °C(lit.) |
| Boiling point: | 90-92 °C(Press: 0.08 Torr) |
| alpha | -25 º (C=6% IN H2O) |
| Density | 1.195±0.06 g/cm3(Predicted) |
| Flash point: | 112 °C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Oil to Semi-Solid |
| pka | 4.36±0.10(Predicted) |
| color | Colourless to Pale Yellow |
| optical activity | [α]20/D 25±1°, c = 6% in H2O |
| BRN | 1720568 |
| InChI | InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1 |
| CAS DataBase Reference | 625-72-9(CAS DataBase Reference) |
| EPA Substance Registry System | Butanoic acid, 3-hydroxy-, (3R)- (625-72-9) |
Safety information for (R)-3-Hydroxybutyric acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (R)-3-Hydroxybutyric acid
| InChIKey | WHBMMWSBFZVSSR-GSVOUGTGSA-N |
| SMILES | C(O)(=O)C[C@H](O)C |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![(-)-1-[5-CHLORO-2-(METHYLAMINO)PHENYL]-1,2,3,4-TETRAHYDROISOQUINOLINE,(-)-TARTRATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB3312661.gif)

You may like
-
 (R)-3-Hydroxybutyric acid CAS 625-72-9View Details
(R)-3-Hydroxybutyric acid CAS 625-72-9View Details
625-72-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1