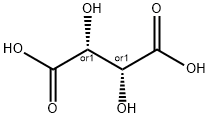
D-(-)-Tartaric Acid
Synonym(s):(2R,3R)-(+)-Tartaric acid;(2S,3S)-(-)-Tartaric acid;L- (+)-Tartaric acid;L -Threaric acid;D-(-)-Tartaric acid
- CAS NO.:147-71-7
- Empirical Formula: C4H6O6
- Molecular Weight: 150.09
- MDL number: MFCD00004238
- EINECS: 205-695-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24

What is D-(-)-Tartaric Acid ?
Chemical properties
D-Tartaric acid is a white crystalline dicarboxylic acid found in many plants, particularly tamarinds and grapes. It is an acidulant that occurs naturally in grapes. It is hygroscopic and rapidly soluble, with a solubility of 150 g in 100 ml of distilled water at 25°c. It has a slightly tarter taste than citric acid, with a tartness equivalent of 0.8–0.9. It augments the flavor of fruits in which it is a natural constituent. It is used in grapeand limeflavored beverages and grape-flavored jellies. It is used as an acidulant in baking powder and as a synergist with antioxidants to prevent rancidity.
The Uses of D-(-)-Tartaric Acid
D-(-)-Tartaric acid is used as a resolving agent in organic synthesis. It is used as a precursor for the preparation of its ester derivatives like D-tartaric acid diethyl ester, D-tartaric acid dimethyl ester and D-tartaric acid diiso-propyl ester. It finds application in the synthesis of chiral aziridine derivative, a common intermediate for the preparation of hydroxyethylamine class HIV protease inhibitors viz. as saquinavir, amprenavir and nelfinavir. It is widely used in the food industry as a beer foaming agent, for food acidity regulations and as a flavoring agent.
Definition
ChEBI: D-tartaric acid is the D-enantiomer of tartaric acid. It has a role as an Escherichia coli metabolite. It is a conjugate acid of a D-tartrate(1-). It is an enantiomer of a L-tartaric acid.
Biotechnological Production
Tartaric acid is generally produced from crude tartar and lees, which are byproducts of wine production. However, there are a few reports of fermentative production of tartaric acid by Gluconobacter suboxydans growing on Glucose or sorbitol. Vanadate plays a central role in this process. The microorganism forms 5-keto-D-gluconic acid, which is oxidized to tartaric acid. The vanadium catalyzes this reaction. Product concentrations up to 2.96 g.L-1 have been observed after 3 days of fermentation.
General Description
D-(-)-Tartaric acid is a polycrystalline solid, widely used as food additive. It has been reported to exhibit piezoelectric effect.
Purification Methods
Crystallise the acid from distilled H2O or *benzene/diethyl ether containing 5% of pet ether (b 60-80o) (1:1). Soxhlet extraction with diethyl ether has been used to remove an impurity absorbing at 265nm. It has also been crystallised from absolute EtOH/hexane and dried in a vacuum for 18hours [Kornblum & Wade J Org Chem 52 5301 1987]. [Beilstein 3 IV 1229.]
Properties of D-(-)-Tartaric Acid
| Melting point: | 172-174 °C(lit.) |
| Boiling point: | 191.59°C (rough estimate) |
| alpha | -12.1 º (c=20, H2O) |
| Density | 1,8 g/cm3 |
| refractive index | -12.5 ° (C=5, H2O) |
| Flash point: | 210 °C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble100mg/mL, clear, colorless |
| form | Crystals or Crystalline Powder |
| pka | 3.0, 4.4(at 25℃) |
| color | White |
| Odor | odorless |
| optical activity | [α]20/D 13.5±0.5°, c = 10% in H2O |
| Water Solubility | 1394 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,9068 |
| BRN | 1725145 |
| Dielectric constant | 35.9(-10℃) |
| Stability: | Stable. Incompatible with oxidizing agents, bases, reducing agents. Combustible. |
| CAS DataBase Reference | 147-71-7(CAS DataBase Reference) |
| NIST Chemistry Reference | D-Tartaric acid(147-71-7) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dihydroxy-, (2S,3S)- (147-71-7) |
Safety information for D-(-)-Tartaric Acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-(-)-Tartaric Acid
| InChIKey | FEWJPZIEWOKRBE-LWMBPPNESA-N |
D-(-)-Tartaric Acid manufacturer
JSK Chemicals
Kaival Chemicals Private Limited
Atharva Trading & Company
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 147-71-7 98%View Details
147-71-7 98%View Details
147-71-7 -
 D-Tartaric Acid 99%View Details
D-Tartaric Acid 99%View Details -
 D-(-)-Tartaric Acid CAS 147-71-7View Details
D-(-)-Tartaric Acid CAS 147-71-7View Details
147-71-7 -
 D-Tartaric acid, puriss CAS 147-71-7View Details
D-Tartaric acid, puriss CAS 147-71-7View Details
147-71-7 -
 D-(−)-Tartaric acid CAS 147-71-7View Details
D-(−)-Tartaric acid CAS 147-71-7View Details
147-71-7 -
 Tartaric Acid CAS 87-69-4View Details
Tartaric Acid CAS 87-69-4View Details
147-71-7 -
 D Tartaric Acid, Pack Type: Drum, Pack Size: 25 KgView Details
D Tartaric Acid, Pack Type: Drum, Pack Size: 25 KgView Details
147-71-7 -
 D-(-)-Tartaric Acid (CAS Number: 147-71-7)View Details
D-(-)-Tartaric Acid (CAS Number: 147-71-7)View Details
147-71-7
