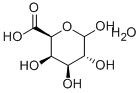
D-GALACTURONIC ACID
- CAS NO.:685-73-4
- Empirical Formula: C6H10O7
- Molecular Weight: 194.14
- MDL number: MFCD00006618
- EINECS: 211-682-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
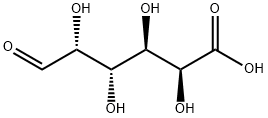
What is D-GALACTURONIC ACID?
Description
D-Galacturonic acid is a sugar acid, an oxidized form of Dgalactose. It is the main component of pectin, in which it exists as the polymer polygalacturonic acid. It has an aldehyde group at C1 and a carboxylic acid group at C6. Other oxidized forms of D-galactose are D-galactonic acid (carboxylic group at C1) and meso-galactaric acid (mucic acid) (carboxylic groups at C1 and C6). It is also a uronic acid or hexuronic acid. Naturally occurring uronic acids are D-glucuronic acid, D-galacturonic acid, L-iduronic acid and D-mannuronic acid.
Description
D-Galacturonic acid (GalA) is an oxidized form of the monosaccharide D-galactose1, a component of the disaccharide lactose. Its structure is sometimes displayed in an open, linear form, with a carboxylic acid group on one end of the chain and an aldehyde on the other; but it is more frequently depicted as a closed aldopyranose. This cyclic form can take two configurations: α-D-galacturonic acid, with the hydroxyl adjacent to the ring oxygen in the axial position (shown here), or the β-epimer with the same hydroxyl in the equatorial position.
Why is GalA significant? It is the primary building block and structure-giving element of pectin2 and other biopolymers found throughout the plant kingdom. The polymeric GalA chains in pectin are connected by α-1,4 glycosidic bonds; some of its carboxyl groups are in the form of methyl esters. Pectin molecules have atomic masses of 200,000 g/mol or higher.
According to the Merck Index, pectin is found in the cell walls of all plant tissues and functions as an intercellular connecting material. It is especially abundant (≈30 wt%) in citrus rinds; but it is also commercially sourced from apples, spinach, sugarbeets, and other fruits and vegetables (see photo). Its main use is as a gelling or filling agent in foods such as jellies, jams, desserts, and candies and as a stabilizer in juices and milk-based drinks.
Henri Braconnot at the Royal Academic Society of Nancy (France) first described pectin in 1824. The primary source of commercial GalA is the hydrolysis of pectin, a process that was first reported by Felix Ehrlich3 at the University of Breslau (Germany; now the University of Wroc?aw [Poland]) in 1917. The process was refined in 2004 by Tetsuya Miyazawa and Toshitaka Funazukuri* at Chuo University (Tokyo). Starting with poly(galacturonic acid) and water with no additives, they used a semibatch flow reactor (220 °C, 10 MPa pressure, 2 min heating time) to obtain a 79% yield of water-soluble products, which were then enzymatically hydrolyzed to GalA and its dimer and trimer.
An internet search showed that worldwide production and consumption of GalA are steadily increasing, but specific numbers are available only in expensive market research reports.
1. CAS Reg. No. 59-23-4.
2. CAS Reg. No. 9000-69-5.
3. Ehrlich was a prominent biochemist. Well before his work on the structure of pectin, he discovered the amino acid isoleucine as a student in 1903.
Chemical properties
The α form melts with decomposition at 159–160C. Soluble in water, slightly soluble in hot alcohol; insoluble in ether
The Uses of D-GALACTURONIC ACID
Biochemical research
Definition
ChEBI: The pyranose form of D-galacturonic acid
Purification Methods
Crystallisation of the acid from 95% EtOH and drying it in a vacuum desiccator (12mm) over P2O5 gives the monohydrate mixture of and anomers (mostly -) as white micro needles, which sinter at ~100-111o and melt at 159-160o, [] D 20 +107o (initial, c 4 in H2O mutarotating to +51o). [Link & Sell Biochemical Preparations 3 74, 78 1953, Beilstein 3 IV 2000.] The -anomer is obtained by warming the -anomer in EtOH, AcOH or EtOAc and has m 1 6 0o (165o, sinters at 1 4 0o), [] D 20 +27o (initial, c 2 in H2O mutarotating to +55.3o in 24hours). The sodium salt [14984-39-5] M 216.1 has [] D 20 +27o (c 10 in H2O after 5hours). The phenylhydrazone has m 141o(from MeOH). [Ehrlich & Schubert Chem Ber 62 1974, 2014 1929, Anderson & King J Chem Soc 5333 1961, Beilstein 3 IV 2001.]
Properties of D-GALACTURONIC ACID
| Melting point: | 166°C |
| Boiling point: | 250.56°C (rough estimate) |
| Density | 1.4301 (rough estimate) |
| refractive index | 1.4455 (estimate) |
| solubility | 295 g/l |
| appearance | hygroscopic white to light yellow crystals or powder |
| pka | 3.30±0.35(Predicted) |
| EPA Substance Registry System | D-Galacturonic acid (685-73-4) |
Safety information for D-GALACTURONIC ACID
Computed Descriptors for D-GALACTURONIC ACID
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 D-Galacturonic acid 98% (HPLC) CAS 685-73-4View Details
D-Galacturonic acid 98% (HPLC) CAS 685-73-4View Details
685-73-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
