Quinestrol
Synonym(s):17α-Ethynyl-1,3,5(10)-estratriene-3,17β-diol 3-cyclopentyl ether;Quinestrol
- CAS NO.:152-43-2
- Empirical Formula: C25H32O2
- Molecular Weight: 364.52
- MDL number: MFCD00079189
- EINECS: 205-803-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
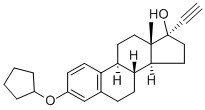
What is Quinestrol?
Absorption
Absorbed following oral administration.
Toxicity
Symptoms of overdose include nausea and vomiting, and withdrawal bleeding may occur in females.
Description
Quinestrol is a synthetic estrogen that is effective in hormone replacement therapy. It is a 3-
The Uses of Quinestrol
Quinestrol is an estrogen-like compound.
The Uses of Quinestrol
antibacterial, antimycoplasmal, isoleucyl-tRNA synthetase inhibitor
What are the applications of Application
Quinestrol is an estrogen-like compound
Indications
Used in hormone replacement therapy, treating symptoms of menopause such as hot flashes. Also used to treat breast and prostate cancer.
Background
The 3-cyclopentyl ether of ethinyl estradiol.
Definition
ChEBI: Quinestrol is a 17-hydroxy steroid and a terminal acetylenic compound. It has a role as a xenoestrogen. It is functionally related to a 17beta-estradiol.
brand name
Estrovis (Parke-Davis).
Pharmacokinetics
Quinestrol is the 3-cyclopentyl ether of ethinyl estradiol (the active metabolite). After gastrointestinal absorption, it is stored in adipose tissue where it is slowly released and metabolized principally to the parent compound, ethinyl estradiol. Ethinyl estradiol is a synthetic derivative of the natural estrogen estradiol.
Metabolism
Metabolized principally to the parent compound, ethinyl estradiol. Ethinyl estradiol is metabolized in the liver. Quantitatively, the major metabolic pathway for ethinyl estradiol, both in rats and in humans, is aromatic hydroxylation, as it is for the natural estrogens.
References
[1]. baumgardner sb, condrea h, daane ta, et al. replacement estrogen therapy for menopausal vasomotor flushes. comparison of quinestrol and conjugated estrogens. obstet gynecol. 1978 apr;51(4):445-52.
[2]. skouby, s.o. criteria for the selection of an optimal estrogen replacement. gynecological endocrinology 15, 60-67 (2001).
[3]. li j, chen f, li c,et al. quinestrol induces spermatogenic apoptosis in vivo via increasing pro-apoptotic proteins in adult male mice. tissue cell. 2014 oct;46(5):318-25.
[4]. li j, chen f, chen y, et al. mitochondrial- and fas-l-mediated pathways involved in quinestrol induced spermatogenic apoptosis in adult rat testes. toxicol mech methods. 2014 dec;24(9):609-15.
Properties of Quinestrol
| Melting point: | 107-108° |
| Boiling point: | 435.69°C (rough estimate) |
| alpha | D25 +5° (c = 0.5 in dioxane) |
| Density | 1.0693 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Dioxane (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.10±0.40(Predicted) |
| color | White to Off-White |
| Merck | 14,8055 |
| CAS DataBase Reference | 152-43-2(CAS DataBase Reference) |
Safety information for Quinestrol
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H350:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Quinestrol
Quinestrol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 17α-Ethynylestradiol 3-cyclopentyl ether CAS 152-43-2View Details
17α-Ethynylestradiol 3-cyclopentyl ether CAS 152-43-2View Details
152-43-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
