Pyrophosphoric acid
Synonym(s):Diphosphoric acid
- CAS NO.:2466-09-3
- Empirical Formula: H4O7P2
- Molecular Weight: 177.98
- MDL number: MFCD00011343
- EINECS: 219-574-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-24 21:11:45
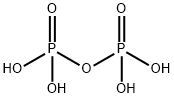
What is Pyrophosphoric acid?
Description
Pyrophosphoric acid, also known under the name
diphosphoric acid, is colorless, odorless, hygroscopic
and is soluble in water, diethyl ether and ethyl alcohol.
It is produced from phosphoric acid by dehydration.
Pyrophosphoric acid slowly hydrolyzes in the presence
of water into phosphoric acid.
H4P2O7 +H2O?2H3PO4
Upon heating, phosphoric acid loses water, being
converted successively, and to some extent concurrently,
into the pyro- and meta-acids:
2H3PO4?H4P2O7 +H2O
nH3PO4?(HPO3)n + [H2O]n
Chemical properties
White to very pale yellow glassy solid
Physical properties
Colorless needles or liquid; hygroscopic. Crystallizes in two anhydrous forms: a metastable form melting at 54.3°C and a second and more stable form melting at 71.5°C; extremely soluble in cold water, reacting very slowly to form phosphoric acid; decomposing much faster in hot water; very soluble in alcohol and ether.
The Uses of Pyrophosphoric acid
Pyrophosphoric Acid has been shown to enhance the rate of pyrophosphate hydrolysis by nonenzymatic catlysis and by inorganic pyrophoshatase. Pyrophosphoric Acid can be used for the preparation of vanadyl pyrophosphato methoxide cluster anion. Pyrophosphoric Acid is used in anion binding of inorganic phosphates due to is ability to form complexes.
The Uses of Pyrophosphoric acid
Catalyst, manufacture of organic phosphate esters, metal treatment, stabilizer for organic per- oxides.
What are the applications of Application
Pyrophosphoric acid is An enhanger of the rate of pyrophosphate hydrolysis by nonenzymatic catlysis and by inorganic pyrophoshatase
Definition
ChEBI: An acyclic phosphorus acid anhydride obtained by condensation of two molecules of phosphoric acid.
Preparation
According to early investigators, the pyro-acid is
formed as low as 100 °C, and the conversion is complete
between 255 and 260 °C. Phosphorous oxychloride
reacts with phosphoric acid in the following manner:
5H3PO4 + POC13?3H4P2O7 + 3HC1
A mixture of the ortho- and meta-acids may be
condensed together to form the pyro-acid by heating on the water bath:
nH3PO4 +[HPO3]n?[H4P2O7]n
Metabolism
Not Available
Properties of Pyrophosphoric acid
| Melting point: | 61 °C |
| Density | approximate 1.9g/ml (25℃) |
| storage temp. | -20°C, Hygroscopic |
| solubility | DMSO (Soluble) |
| form | white crystals |
| pka | 0.99±0.10(Predicted) |
| color | white crystals, crystalline |
| Water Solubility | 709g/100mL H2O (23°C); soluble alcohol, ether [MER06] |
| Merck | 13,8098 |
| Stability: | Hygroscopic, Moisture Sensitive |
| CAS DataBase Reference | 2466-09-3(CAS DataBase Reference) |
| EPA Substance Registry System | Diphosphoric acid (2466-09-3) |
Safety information for Pyrophosphoric acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyrophosphoric acid
| InChIKey | XPPKVPWEQAFLFU-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
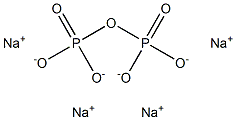
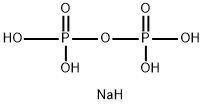
 Diphosphoric acid CAS 2466-09-3View Details
Diphosphoric acid CAS 2466-09-3View Details
2466-09-3 -
 Pyrophosphoric acid CAS 2466-09-3View Details
Pyrophosphoric acid CAS 2466-09-3View Details
2466-09-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4