Pyridoxal 5’-Phosphate
- CAS NO.:853645-22-4
- Empirical Formula: C8H10NO6P
- Molecular Weight: 247.14
- MDL number: MFCD00006333
- EINECS: 200-208-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:03:11
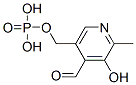
What is Pyridoxal 5’-Phosphate?
Description
Pyridoxal 5-phosphate—the bioactive form of vitamin B6—is a cofactor in many enzymatic reactions. It was first prepared in 1944 by I. C. Gunsalus and co-workers at Cornell University. Some people claim that Vitamin B6?can alleviate symptoms of pregnant women’s morning sickness and alcoholic hangovers.
The Uses of Pyridoxal 5’-Phosphate
Enzyme cofactor.Normal coenzyme form of Vitamin B6
The Uses of Pyridoxal 5’-Phosphate
Pyridoxal 5′-phosphate hydrate has also been used:
- as a reference standard to quantify vitamin B6 in feed and digesta samples using high performance liquid chromatography (HPLC)
- in D-amino acid transaminase reaction(10)
- as a cofactor for L-glutamic acid decarboxylase
Definition
ChEBI: Pyridoxal 5'-phosphate is the monophosphate ester obtained by condensation of phosphoric acid with the primary hydroxy group of pyridoxal. It has a role as a coenzyme, a human metabolite, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, a mouse metabolite, an EC 2.7.7.7 (DNA-directed DNA polymerase) inhibitor and a cofactor. It is a vitamin B6 phosphate, a member of methylpyridines, a monohydroxypyridine and a pyridinecarbaldehyde. It is functionally related to a pyridoxal. It is a conjugate acid of a pyridoxal 5'-phosphate(2-).
General Description
Pyridoxal 5′-phosphate (PLP) is synthesized in a multiple-step process. The two pathways inlcude pyridoxal phosphate biosynthetic protein (PdxA)- pyridoxine-5′-phosphate synthase (PdxJ) pathway and the pyridoxal 5′-phosphate synthase subunit PDX1/PDX2 pathway. It is the active form of pyridoxine.
Biochem/physiol Actions
Pyridoxal 5′-phosphate (PLP) aids in carbohydrate and fat metabolism by serving as a cofactor. It is majorly responsible for catalyzing the enzymatic reactions involved in sphingolipid synthesis and neurotransmitter (dopamine and serotonin) synthesis. PLP is used in the studies of PLP-dependent enzyme active sites. PLP is also a cofactor for a wide range of enzymes including mitochondrial 5-Aminolevulinic acid synthase (ALAS) cysteine desulfurase, cystathionine γ-synthase (CGS), ornithine 4,5-aminomutase (OAM), and D-serine dehydratase.
Biotechnological Applications
Pyridoxal 5’-Phosphate(PLP) is also often employed as an inhibitor and site-directed, conformationally sensitive reporter group for studying the environment of reactive amino groups within proteins. PLP forms aldimine adducts that can be reduced with nucleophilic reducing reagents such as sodium borohydride or sodium cyanoborohydride. Solid PLP is light-sensitive, and solutions should be prepared fresh. There are four pKa values associated with this coenzyme: a pKa < 2.5 and another at 6.20 for the phosphate group, a pKa of 4.14 for the hydroxyl group, and a pKa of 8.69 for the pyridinium group.
Synthesis
A process for the synthesis ofpyridoxal5' -phosphate, comprising the steps of:
a) adding phenetidine into pyridoxal solution, and reacting to generate pyridoxal Schiff base;
b) adding pyridoxal Schiff base into ionic liquid, and stirring until the pyridoxal Schiff base is fully dissolved to obtain an ion mixed solution; adding pyridoxal Schiff base into ionic liquid, stirring at 35-45 ℃, and cooling the
obtained ionic mixed solution to room temperature after the pyridoxal Schiff base is completely dissolved;
c) adding polyphosphoric acid into the ion mixed solution in which the pyridoxal Schiff base is dissolved, stirring for reaction, cooling after the
reaction is finished, and separating out solids from the ion mixed
solution; filtering solid particles separated out from the ion mixed
solution, wherein the solid obtained by filtering is pyridoxal 5' -phosphate Schiff base, and the obtained liquid is phosphorus-containing ionic liquid; the weight ratio of pyridoxal Schiff base dissolved in the ion mixed solution to the polyphosphoric
acid is within the range of 1:1-1:3, the reaction time is within the
range of 8-12h, the reaction temperature is within the range of 25-40 ℃,
and the temperature is reduced to 10 ℃ after the reaction is finished;
the phosphorus-containing ionic liquid obtained by filtering can be
recycled;
d) carrying out hydrolysis purification reaction on the pyridoxal 5 '-phosphate Schiff base obtained in the step c) to obtain a final product pyridoxal 5' -phosphate.
Enzyme inhibitor
This vitamin B6-derived coenzyme, often abbreviated PLP, plays a central role in metabolism.
In addition to facilitating aminotransfer reactions, PLP is a coenzyme in reactions involving:
(a) loss of the a-proton, resulting in racemization, cyclization, or b-elimination/replacement (e.g., alanine racemase, 1- aminocyclopropane-1-carboxylate synthase, and serine dehydratase, respectively);
(b) loss of the a-carboxylate as carbon dioxide (e.g., glutamate decarboxylase);
(c) removal/replacement of a group by aldol cleavage (e.g., threonine aldolase);
(d) catalysis via ketimine intermediates (e.g., selenocysteine lyase).
Properties of Pyridoxal 5’-Phosphate
| Melting point: | 140-143 ºC |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly), Water (Slightly) |
| form | powder |
| pka | pKa 8.69(H2O
t = 25
I = 0.15)(Approximate) |
| color | White to Pale Yellow |
| Odor | Odorless |
| BRN | 234749 |
| Stability: | Hygroscopic |
Safety information for Pyridoxal 5’-Phosphate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyridoxal 5’-Phosphate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Pyridoxal phosphate 97% CAS 853645-22-4View Details
Pyridoxal phosphate 97% CAS 853645-22-4View Details
853645-22-4 -
 Pyridoxal 5′-phosphate hydrate CAS 853645-22-4View Details
Pyridoxal 5′-phosphate hydrate CAS 853645-22-4View Details
853645-22-4 -
 Pyridoxal 5′-phosphate hydrate CAS 853645-22-4View Details
Pyridoxal 5′-phosphate hydrate CAS 853645-22-4View Details
853645-22-4 -
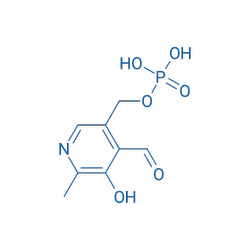 CAS 54 47 7 PYRIDOXAL 5 PHOSPHATE, Strength: 50 mg, Packaging Type: StripView Details
CAS 54 47 7 PYRIDOXAL 5 PHOSPHATE, Strength: 50 mg, Packaging Type: StripView Details
853645-22-4 -
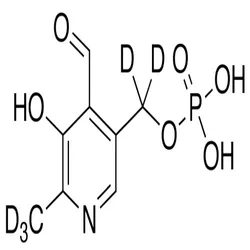 CAS 54 47 7 PYRIDOXAL 5 PHOSPHATE, Strength: 50 mg, Packaging Type: BoxView Details
CAS 54 47 7 PYRIDOXAL 5 PHOSPHATE, Strength: 50 mg, Packaging Type: BoxView Details
853645-22-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
