Sodium Phosphate Monobasic Monohydrate
Synonym(s):phosphate buffer;Monosodium phosphate;Sodium phosphate monobasic monohydrate;Sodium dihydrogen phosphate monohydrate;NaH?PO?
- CAS NO.:10049-21-5
- Empirical Formula: H4NaO5P
- Molecular Weight: 137.992291
- MDL number: MFCD00081857
- EINECS: 600-102-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
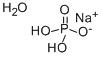
What is Sodium Phosphate Monobasic Monohydrate?
Description
Sodium Phosphate Monobasic Monohydrate is a reagent with very high buffering capacity widely used in molecular biology, biochemistry and chromatography. it can be used as a buffer to adjust pH. In medicine, it is sometimes used as a stimulant laxative before certain operations and medical procedures. Sodium Phosphate Monobasic Monohydrate is often used in foods and in water treatment. It is used as sequestrant, emulsifier, mordant in dyeing, reagent and buffer in foods and analytical chemistry. It is applied as a fireproofing agent and for weighting silk in tanning. It is employed in manufacturing of enamels, ceramics, detergents, boiler compounds, in soldering and brazing instead of borax.
Monobasic sodium phosphate is used in baking powders, acid cleansers, electroplating, as a dry acidulant, and in treating boiler water.
Chemical properties
Anhydrous salt: white crystalline powder; slightly hygroscopic; forms sodium acid pyrophosphate, Na2H2P2O7 on heating above 225°C and sodium metaphosphate (NaPO3)n at about 350 to 400°C; very soluble in water, aqueous solution acidic.
Monohydrate: white orthorhombic crystals or granules; density 2.04 g/cm3 ; loses its water of crystallization at 100°C; very soluble in water, pH of 1% solution 4.5; insoluble in alcohol.
Dihydrate: large transparent crystals; orthorhombic bisphenoidal structure; density 1.915 g/cm 3 ; decomposes at 60°C; very soluble in water; insoluble in alcohol.
Chemical properties
The USP 32 states that monobasic sodium phosphate contains one
or two molecules of water of hydration or is anhydrous.
The hydrated forms of monobasic sodium phosphate occur as
odorless, colorless or white, slightly deliquescent crystals. The
anhydrous form occurs as a white crystalline powder or granules.
The Uses of Sodium Phosphate Monobasic Monohydrate
Sodium Phosphate Monobasic Monohydrate is often used in foods and in water treatment. it can be used as a buffer to adjust pH. In medicine, it is sometimes used as a stimulant laxative before certain operations and medical procedures.
The Uses of Sodium Phosphate Monobasic Monohydrate
Excipient.
The Uses of Sodium Phosphate Monobasic Monohydrate
Sodium phosphate monobasic monohydrate has been used for the preparation of cardioplegia solution. It has also been used as a component in McIlvaine′s buffer and MgCl2 buffer.
What are the applications of Application
Sodium Phosphate, Monobasic, Monohydrate is a reagent with very high buffering capacity for formulating screens or for optimization.
What are the applications of Application
Sodium Phosphate, Monobasic, Monohydrate, Cell Culture Grade is a reagent with a high buffering capacity
Preparation
Monobasic sodium phosphate can be prepared by partial neutralization of phosphoric acid with sodium hydroxide in equimolar amounts:
H3PO4+ NaOH →NaH2PO4+ H2O
It also can be made by treating disodium hydrogen phosphate with phosphoric acid in proper stoichiometric amount:
Na2HPO4+ H3PO4→2NaH2PO4
Production Methods
Monobasic sodium phosphate is prepared by adding phosphoric acid to a hot, concentrated solution of disodium phosphate until the liquid ceases to form a precipitate with barium chloride. This solution is then concentrated and the monobasic sodium phosphate is crystallized.
Definition
ChEBI: Sodium dihydrogenphosphate monohydrate is a hydrate that is the monohydrate form of sodium dihydrogenphosphate. It contains a sodium dihydrogenphosphate.
General Description
Useful in conjuction with sodium phosphate, dibasic (Cat. No. 567550) in the preparation of biological buffers(absorbance: ≤0.01 at 260 nm and 280 nm).
Pharmaceutical Applications
Monobasic sodium phosphate is used in a wide variety of
pharmaceutical formulations as a buffering agent and as a
sequestering agent. Therapeutically, monobasic sodium phosphate
is used as a mild saline laxative and in the treatment of hypophosphatemia.
Monobasic sodium phosphate is also used in food products, for
example, in baking powders, and as a dry acidulant and
sequestrant.
Safety
Monobasic sodium phosphate is widely used as an excipient in
parenteral, oral, and topical pharmaceutical formulations.
Phosphate occurs extensively in the body and is involved in
many physiological processes since it is the principal anion of
intracellular fluid. Most foods contain adequate amounts of
phosphate, making hypophosphatemia virtually unknown except
in certain disease states or in patients receiving total parenteral
nutrition. Treatment is usually by the oral administration of up to
100 mmol of phosphate daily.
Approximately two-thirds of ingested phosphate is absorbed
from the gastrointestinal tract, virtually all of it being excreted in the
urine, and the remainder is excreted in the feces.
Excessive administration of phosphate, particularly intravenously,
rectally, or in patients with renal failure, can cause
hyperphosphatemia that may lead to hypocalcemia or other severe
electrolyte imbalances. Adverse effects occur less frequently
following oral consumption, although phosphates act as mild saline
laxatives when administered orally or rectally (2–4 g of monobasic
sodium phosphate in an aqueous solution is used as a laxative).
Consequently, gastrointestinal disturbances including diarrhea,
nausea, and vomiting may occur following the use of monobasic
sodium phosphate as an excipient in oral formulations. However,
the level of monobasic sodium phosphate used as an excipient in a pharmaceutical formulation is not usually associated with adverse
effects.
LD50 (rat, IM): 0.25 g/kg(10)
LD50 (rat, oral): 8.29 g/kg
storage
Monobasic sodium phosphate is chemically stable, although it is
slightly deliquescent. On heating at 100°C, the dihydrate loses all of
its water of crystallization. On further heating, it melts with
decomposition at 205℃, forming sodium hydrogen pyrophosphate,
Na2H2P2O7. At 250℃ it leaves a final residue of sodium
metaphosphate, NaPO3.
Aqueous solutions are stable and may be sterilized by autoclaving.
Monobasic sodium phosphate should be stored in an airtight
container in a cool, dry place.
Incompatibilities
Monobasic sodium phosphate is an acid salt and is therefore
generally incompatible with alkaline materials and carbonates;
aqueous solutions of monobasic sodium phosphate are acidic and
will cause carbonates to effervesce.
Monobasic sodium phosphate should not be administered
concomitantly with aluminum, calcium, or magnesium salts since
they bind phosphate and could impair its absorption from the
gastrointestinal tract. Interaction between calcium and phosphate,
leading to the formation of insoluble calcium phosphate precipitates,
is possible in parenteral admixtures.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections; infusions; ophthalmic, oral, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sodium Phosphate Monobasic Monohydrate
| Melting point: | 100°C -H₂O |
| Boiling point: | 399 °C |
| Density | 2,04 g/cm3 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M, clear, colorless |
| form | Solid |
| color | White semi-transparentor |
| Odor | Odorless |
| PH | 4.1-4.5 (25℃, 50mg/mL in H2O) |
| PH Range | 4.1 - 4.5 at 50 g/l at 25 °C |
| Water Solubility | Soluble in water; insoluble in ethanol, ether and chloroform. |
| λmax | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.02 |
| Sensitive | Hygroscopic |
| Merck | 14,8660 |
| CAS DataBase Reference | 10049-21-5(CAS DataBase Reference) |
| EPA Substance Registry System | Monosodium phosphate monohydrate (10049-21-5) |
Safety information for Sodium Phosphate Monobasic Monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Sodium Phosphate Monobasic Monohydrate
| InChIKey | BBMHARZCALWXSL-UHFFFAOYSA-M |
Sodium Phosphate Monobasic Monohydrate manufacturer
JSK Chemicals
Buradon Inc.
Halogens
New Products
1-Boc-4-cyanopiperidine 1,3-Cyclopentanedione TETRABUTYLAMMONIUM CYANIDE 3-Pyridineacrylic acid TETRAHYDRO-2H-PYRAN-3-OL tert-Butyl carbazate 3-(Chloromethyl)pyridine 4-(Chloromethyl)benzoyl chloride, 97% 1,3,5-Trimethoxybenzene, 98% N-Benzyloxycarbonyl-L-proline, 95% ß-Citronellol, 95% Aluminum potassium sulfate dodecahydrate Methyl-2-Methoxy-5-Sulfamoyl Benzoate 2,3,5-Triiodobenzoic Acid 2-Chloro Acetophenone Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) Para Chloro Toluene (PCT) Diiodo Pentoxide 1-(Sulfamoylamino)Propane 1-phenyl-1,2,3,4-tetrahydroisoquinoline 2-amino benzyl alcohol Pyridine-2-carboxaldehyde Veratric acid 3,5-benzyloxy AcetophenoneRelated products of tetrahydrofuran
You may like
-
 Sodium dihydrogen phosphate monohydrate 98%View Details
Sodium dihydrogen phosphate monohydrate 98%View Details -
 Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
10049-21-5 -
 Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
10049-21-5 -
 Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
10049-21-5 -
 Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
10049-21-5 -
 Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
Sodium dihydrogen phosphate monohydrate CAS 10049-21-5View Details
10049-21-5 -
 Sodium Phosphate Monobasic Monohydrate for HPLC CAS 10049-21-5View Details
Sodium Phosphate Monobasic Monohydrate for HPLC CAS 10049-21-5View Details
10049-21-5 -
 Sodium Phosphate Monobasic Monohydrate extrapure AR CAS 10049-21-5View Details
Sodium Phosphate Monobasic Monohydrate extrapure AR CAS 10049-21-5View Details
10049-21-5
