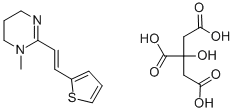
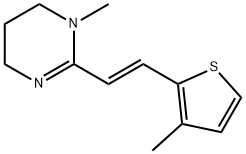
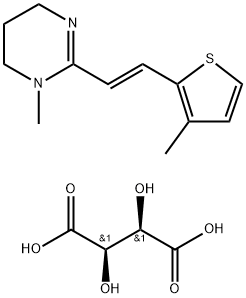
pyrante
- CAS NO.:15686-83-6
- Empirical Formula: C11H14N2S
- Molecular Weight: 206.31
- MDL number: MFCD00242767
- EINECS: 2397741
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-09 19:38:33
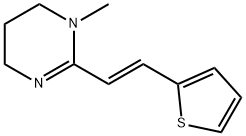
What is pyrante?
Absorption
Pyrantel is poorly absorbed from the GI tract of humans , .
Peak serum concentrations occur 1–3 hours after a single dose .
Toxicity
Mild adverse effects include nausea, vomiting, diarrhea, headache, and dizziness .
LD50 in rats is 535 mg/kg .
Reported effects in humans in case of overdose include gastrointestinal disturbance, central nervous system effects, and superficial skin reactions. In one study, serum aspartate aminotransferase (AST) and serum alanine-aminotransferase (ALT) values were increased in approximately 2% of patients .
Pyrantel should be used with caution in patients with severe malnutrition or anemia. Supportive therapy is recommended for anemic, dehydrated, or malnourished patients before administration of the drug .
Pyrantel pamoate has been placed in pregnancy category C. This refers to the fact that animal studies have revealed adverse effects on the fetus (teratogenic/embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus .Data on the use of pyrantel pamoate in pregnant women are quite limited. In mass treatment programs for which the World Health Organization (WHO) has observed that the benefits of treatment outweigh the risks, WHO allows the use of pyrantel pamoate in the 2nd and 3rd trimesters of pregnancy, due to the fact that the effects of pyrantel on birth outcome are uncertain. The risk of treatment in pregnant women already known to have an infection needs to be balanced with the risk of disease progression if treatment were to be omitted . Individuals with liver disease are more susceptible to the toxicity in cases of pyrantel overexposure , .
There are no data regarding the presence of pyrantel in breast milk. Pyrantel is poorly absorbed from the GI tract; therefore, excretion into breast milk may be minimal. Some experts recommend that a single dose of pyrantel therapy may be given to breastfeeding women .
The Uses of pyrante
Anthelmintic.
Indications
For the treatment of enterobiasis including roundworm (ascariasis), pinworm (enterobius) and hookworm (strongyloides) and hookworm (ancylostoma) in the pyrantel pamoate form .
Pyrantel is available in various formulations for humans, dogs, and cats as the pamoate (US Pharmacopeia nomenclature) or embonate (European Pharmacopoeia nomenclature) salt, which contains 34.7% pyrantel base combined with pamoic acid . , .
Pyrantel pamoate (embonate) ingested orally is effective for removal and control of ascarid and hookworm infections in puppies and dogs (adult Toxocara canis, Toxascaris leonina, Ancylostoma tubaeforme, An. braziliense, Uncinaria stenocephala), cats (adult Toxocara cati, Toxa. leonina, An. caninum, An. braziliense, U. stenocephala), horses and ponies (adult and immature Parascaris equorum, adult Strongylus vulgaris, S. edentatus, S. equinus, Cyathostomes (Triodontophorus spp., Cyathostomum spp., Cylicodontophorus spp., Cylicocyclus spp., Cylicostephanus spp., Poteriostomum spp.), Oxyuris equi, Anoplocephala perfoliata), swine (adult Ascaris suum, Oesophagostomum dentatum), and humans (adult A. lumbricoides, Enterobius vermicularis, An. duodenale, Necator americanus) .
Background
Pyrantel is a pyrimidine-derivative anthelmintic agent for the oral treatment of various parasitic worm infections including ascariasis, hookworm infections, enterobiasis (pinworm infection), trichostrongyliasis, and trichinellosis .
Pyrantel was initially described in 1965 by researchers from Pfizer who sought cyclic amidines with suitable pharmacokinetic properties (specifically, duration of action) for use as an anthelmintic drug. Pyrantel is mainly available in formulations for dogs and cats as the embonate salt, containing a 34.7% pyrantel base .
Pyrantel is on the World Health Organization's List of Essential Medicines, which are the safest and most effective medicines required in a functioning health system , .
A depolarizing neuromuscular-blocking agent causing longstanding nicotinic receptor activation, resulting in spastic paralysis of susceptible nematodes (worms). Pyrantel has shown to be effective after a single dose .
In humans, it is administered as pyrantel pamoate ,,,.
Definition
ChEBI: A carboxamidine that is 1,4,5,6-tetrahydropyrimidine that is substituted at position 1 by a methyl group and at position 2 by an (E)-2-(2-thienyl)vinyl group. It is used, particularly as the embonate [4,4'-methylenebis(3-hydroxy-2-naphthoa e)] salt, as an anthelmintic that is effective against intestinal nematodes including threadworms, roundworms and hookworms, and is included in the WHO 'Model List of Essential Medicines'.
brand name
Banminth (Pfizer).
Pharmacokinetics
It has similar properties to both competitive and depolarizing neuromuscular blocking agents, which leads to the understanding of the paralytic effect of the drug has on parasites, ultimately resulting in the death of the parasite , .
Metabolism
Pyrantel is administered orally. The poor solubility of the pamoate salt offers the advantage of reduced absorption from the gastrointestinal tract and allows the drug to reach and act against parasites in the large intestine. Metabolism of pyrantel is rapid . The absorbed drug is partly metabolized in the liver .
Properties of pyrante
| Melting point: | 178-179° |
| Boiling point: | 324.4±44.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | 12.60±0.20(Predicted) |
Safety information for pyrante
Computed Descriptors for pyrante
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2