Prussian Blue
Synonym(s):Prussian blue
- CAS NO.:14038-43-8
- Empirical Formula: C6FeN6.4/3Fe
- Molecular Weight: 859.23
- MDL number: MFCD00135663
- EINECS: 237-875-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:43
What is Prussian Blue?
Absorption
It is poorly or not absorbed from the gastrointestinal tract walls after oral ingestion. Systemic absorption is assumed to be insignificant, with minimal release of cyanide from the complex. A small amount (approximately 2%) of the hexacyanoferrate ion was absorbed after oral ingestion of prussian blue but with no signs of decomposition. Prussian blue is not systemically bioavailable .
Toxicity
Mild cases of hypokalemia have been reported as prussian blue may bind other electrolytes found in the gastrointestinal tract. Gastrointestinal symptoms include abdominal pain or distension. Constipation may occur resulting in further decreased gastrointestinal motility and increased reabsorption and exposure time to radioisotopes, but may may be treated with a fiber based laxative and/or a high fiber diet. Oral dose that results in acute toxicity in mouse, rat and rabbit is >8000mg/kg. Based on reported adverse events and mechanism of action, possible overdose symptoms may include obstipation, obstruction, or severe decrease in electrolytes.
Description
Iron blue is chemically referred to as ferric ammonium ferrocyanide Fe(Fe(CN)6)3. This material is generated through the reaction of sodium ferrocyanide and ferrous sulfate in the presence of ammonium sulfate. Pigments prepared with sodium or potassium salts are called ferric ferrocyanide.
Description
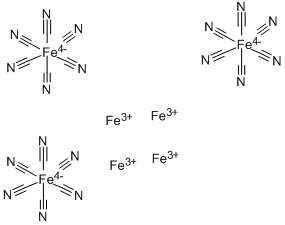
The pigment Prussian blue consists of iron cations, cyanide anions, and water. The empirical formula—minus the water of crystallization—is Fe7(CN)18. This seems odd with respect to the iron oxidation state until you learn that the complex contains Fe(II) and Fe(III). Thus, the formula that gives a truer idea of its composition is Fe4[Fe(CN)6]3. Its formal name is iron(III) hexacyanoferrate(II).
As shown in the two left-hand drawings, the Fe(CN)6?anion in Prussian blue is octahedral. The right-hand?drawing shows its unit cell, which has a cubic lattice structure.?In addition to having as many as 16 molecules of water per formula unit, the compound usually contains inorganic impurities, which can affect its color.
The name Prussian blue originated in the 18th century, when the compound was used to dye the uniform coats for the Prussian army. Over the years, the pigment acquired several other “blue” names, including Berlin, Parisian, and Turnbull’s blue. It has been used for centuries in unusually diverse applications (see the information box). Despite the presence of cyanide groups, the pigment is not toxic to humans.
Seniors among us will recognize “Prussian blue” as a crayon color. Prussian blue was one of the 38 original Crayola colors introduced by Binney & Smith Inc. in 1903. (The company name was subsequently changed to Crayola; later, the firm was acquired by Hallmark.)
The Prussian blue crayon name lasted until 1958, when it was changed to midnight blue. The reason for the change is unclear: One source says it was made because no one knew what Prussia was anymore; another reports that the move was spurred by political correctness during the Cold War.
Chemical properties
dark blue crystalline powder
The Uses of Prussian Blue
Prussian blue (KFe(Fe(CN)6)) is an intense reddish blue pigment with fairly good properties. It is used as a coloring pigment in many types of paint systems and is also used in the production of lead chrome greens.
The Uses of Prussian Blue
Iron(III) hexacyanoferrate(II) is uses extensively as an Iron Oxide dye. Prussian blue is used as a paint and wallpaper printing, chemical coatings, carbon paper and in the plastics industry. as an antidote for poisoning with radioactive cesium or thallium. In the metalworking and mechanical engineering Prussian blue is thinly applied as a paste on metal surfaces in order to assess the quality scraped surfaces can.
Indications
Indicated for treatment of patients with known or suspected internal contamination with radioactive cesium and/or radioactive or non-radioactive thallium to increase their rates of elimination.
Background
Prussian blue is described as a deep blue pigment that is produced when the oxidation of ferrous ferrocyanide salts occurs. It contains ferric hexacyanoferrate(II) in a cubic lattice crystal structure. It is insoluble in water but also tends to form a colloid thus can exist in either colloidal or water-soluble form, and an insoluble form. It is orally administered for clinical purposes to be used as an antidote for certain kinds of heavy metal poisoning, such as thallium and radioactive isotopes of caesium. Prussian blue is included in the World Health Organization Model List of Essential Medicines as a specific antidote used in poisonings to provide symptomatic and supportive treatment. It was also administered in individuals exposed to 137-Cs+ during Goiania accident, one of the worst radioactive contamination incidents that occured in Brazil, 1983.
What are the applications of Application
Iron(III) hexacyanoferrate(II) is an Iron Oxide dye
Definition
The most common and best- known name for blue iron ferrocyanide (iron blue) pigments made by a variety of procedures.
Origin
Ferric hexacyanoferrate(II), known as Prussian blue, has the empirical formula Fe4[Fe(CN)6]3. It was probably synthesized for the first time by the paint maker Diesbach in Berlin in 1704, and it was one of the first synthetic pigments. A number of different, even if chemically related compounds, are named Prussian blue. Here this term refers only to insoluble ferric hexacyanoferrate(II)[1].
Flammability and Explosibility
Not classified
Pharmacokinetics
Prussian blue is an insoluble radioactive metals chelating agent and absorbent. It acts by ion-exchange, adsorption, and mechanical trapping within the crystal structure and has a very high affinity for radioactive and non-radioactive cesium and thallium. The antidote therapy greatly minimizes the extent of contamination and reduces the half life of radioactive isotopes which have relatively long physicall half life and uniform tissue distribution. Data suggest that in humans, Prussian blue can reduce cesium’s half-life by approximately 43% and reduce total body burdens by significantly increasing the feces-to-urine excretion ratio .
Metabolism
No evidence of decomposition after oral ingestion . Prussian blue does not undergo hepatic metabolism; use of the drug is not contraindicated in patients with hepatic impairment .
Structure and conformation
Prussian blue has a basic cubic structure consisting of alternating iron(II) and iron(III) located on a face centered cubic lattice, in such a way that the iron(III) ions are surrounded octahedrically by nitrogen atoms, and iron(II) ions are surrounded by carbon atoms[2].
References
[1] Crisponi, G. and V. Nurchi. “Chelating Agents as Therapeutic Compounds—Basic Principles.” Chelation Therapy in the Treatment of Metal Intoxication (2016) 35-61.
[2] Karyakin, A. “Chemical and biological sensors based on electroactive inorganic polycrystals.”Electrochemical Sensors, Biosensors and their Biomedical Applications (2008) 411-439.
Properties of Prussian Blue
| Density | 1.8 |
| storage temp. | Room Temperature |
| solubility | insoluble in H2O, dilute acid solutions, organic solvents |
| form | Powder |
| color | Dark blue |
| Water Solubility | practically insoluble |
| Sensitive | Hygroscopic |
| Merck | 14,7910 |
| Solubility Product Constant (Ksp) | pKsp: 40.52 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents, ammonia. Light sensitive. |
| InChI | InChI=1S/18CN.7Fe/c18*1-2;;;;;;;/q;;;;;;;;;;;;;;;;;;3*-4;4*+3 |
| EPA Substance Registry System | Iron ferrocyanide (Fe4[Fe(CN)6]3) (14038-43-8) |
Safety information for Prussian Blue
Computed Descriptors for Prussian Blue
| InChIKey | DNMNDNSFJMUUFM-UHFFFAOYSA-N |
| SMILES | [Fe-4](C#N)(C#N)(C#N)(C#N)(C#N)C#N.[Fe-4](C#N)(C#N)(C#N)(C#N)(C#N)C#N.[Fe-4](C#N)(C#N)(C#N)(C#N)(C#N)C#N.[Fe+3].[Fe+3].[Fe+3].[Fe+3] |
Prussian Blue manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


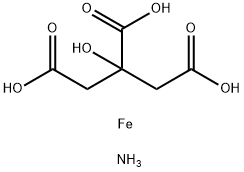


You may like
-
 14038-43-8 99%View Details
14038-43-8 99%View Details
14038-43-8 -
 Iron(III) hexacyanoferrate(II) CAS 14038-43-8View Details
Iron(III) hexacyanoferrate(II) CAS 14038-43-8View Details
14038-43-8 -
 Iron(III) hexacyanoferrate(II) CAS 14038-43-8View Details
Iron(III) hexacyanoferrate(II) CAS 14038-43-8View Details
14038-43-8 -
 Iron(III) ferrocyanide 99%View Details
Iron(III) ferrocyanide 99%View Details
14038-43-8 -
 Iron(III) Hexacyanoferrate(II) CAS 14038-43-8View Details
Iron(III) Hexacyanoferrate(II) CAS 14038-43-8View Details
14038-43-8 -
 Iron(III) ferrocyanide CAS 14038-43-8View Details
Iron(III) ferrocyanide CAS 14038-43-8View Details
14038-43-8 -
 Prussian Blue Pigment Powder, LooseView Details
Prussian Blue Pigment Powder, LooseView Details
14038-43-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
