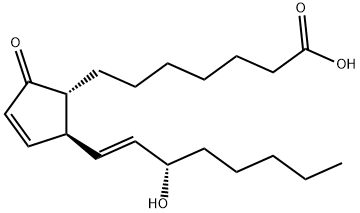
PROSTAGLANDIN A1
Synonym(s):(13E,15S)-15-Hydroxy-9-oxoprosta-10,13-dien-1-oic acid;7-[(1R,2S)-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopent-3-en-1-yl]heptanoic acid;PGA1
- CAS NO.:14152-28-4
- Empirical Formula: C20H32O4
- Molecular Weight: 336.47
- MDL number: MFCD00077858
- EINECS: 682-017-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:01:57
What is PROSTAGLANDIN A1?
Description
Prostaglandin A1 (PGA1) was first isolated as a dehydration product of the PGE1 compounds found in human semen. It has been shown to cause renal vasodilation, increased urine sodium excretion, and lowered arterial pressure in hypertensive patients. It also has growth-
The Uses of PROSTAGLANDIN A1
Prostaglandin A1 has been shown to inhibit human immunodeficiency virus type 1 (HIV-1) replication in various cell types. In addition, as a precursor to the anti-inflammatory prostaglandins, it activates pro-inflammatory PGD2 receptor CRTH2 initiating the anti-inflammatory response.
What are the applications of Application
PGA1 (Prostaglandin A1) is a potent anti-inflammatory cyclopentenone prostaglandin demonstrating inhibition of NFκB
Definition
ChEBI: Prostaglandin A1 is a prostaglandins A. It is a conjugate acid of a prostaglandin A1(1-).
General Description
Prostaglandin A1(PGA1) belongs to the class of cyclopentenone prostaglandin. PGA1 is generated by the dehydration of prostaglandin E1 (PGE1).
Biochem/physiol Actions
Prostaglandin A1 (PGA1) boosts the expression of human liver-specific cytochrome P450 4F3B (CYP4F3B) and stimulates the synthesis of 20-hydroxy-eicosatetraenoic acids (HETEs). It has an ability to stimulate cell differentiation, ferritin synthesis and heat shock response in human cells. PGA1 exhibits antitumor and antiviral activities. In addition, it also elicits anti-inflammatory effects by activating peroxisome proliferator-activated receptor (PPAR). Elevated levels of PGA1 has been observed in hypertension patients.
Enzyme inhibitor
This eicosanoid (FWfree-acid = 336.47 g/mol; CAS 14152-28-4; Symbol: PGA1) induces apoptosis and stimulates the expression of stress genes. The structure and role of the prostaglandin A1 was investigated by Bergstr?m and Samuelsson, who shared the 1982 Nobel Prize in Physiology or Medicine for their pioneering work on the eicosanoids. Target(s): CYP4F2; DNA topoisomerase II; HIV-1 transcription; 3ahydroxysteroid dehydrogenase, NADP+-dependent; IkB kinase; Mayaro virus replication; stress-induced NF-kB activation; and virus protein biosynthesis, or translation.
References
1) Lee et al. (1972) Renal effects of prostaglandin A1 in patients with essential hypertension; Kidney Int. 1 254 2) Toppozada et al. (1988) Treatment of preeclampsia with prostaglandin A1; Am. J. Obstet. Gynecol. 159 160 3) Rossi et al. (2000) Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase; Nature 403 103 4) Mandal et al. (2005), Yin-yang: balancing act of prostaglandins with opposing functions to regulate inflammation; J. Immunol. 175 6271 5) Carta et al. (2014) Prostaglandin A1 inhibits avian influenza virus replication at a postentry level: Effect on virus protein synthesis and NF-kB activity; Prostaglandins Leukot. Essential Fatty Acids 91 311 6) Tsukimoto et al. (2015) A new role for PGA1 in inhibiting hepatitis C virus-IRES-mediated translation by targeting viral translation factors; Antiviral Res. 117 1 7) Diez-Dacal et al. (2011) Identification of aldo-keto reductase AKR1B10 as a selective target for modification and inhibition by prostaglandin A(1): implications for antitumoral activity; Cancer Res. 71 4161 8) Anta et al. (2016), PGA1-induced apoptosis involves specific activation of H-Ras and N-Ras in cellular endomembranes; Cell Death Dis. 7 e2311
Properties of PROSTAGLANDIN A1
| Melting point: | 43°C |
| Boiling point: | 392.93°C (rough estimate) |
| Density | 1.0192 (rough estimate) |
| refractive index | 1.4434 (estimate) |
| storage temp. | -20°C |
| solubility | acetone: 10 mg/mL, clear |
| form | White solid. |
| pka | 4.77±0.10(Predicted) |
| color | Off-white |
| BRN | 2003401 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
Safety information for PROSTAGLANDIN A1
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for PROSTAGLANDIN A1
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Prostaglandin A1 CAS 14152-28-4View Details
Prostaglandin A1 CAS 14152-28-4View Details
14152-28-4 -
 Prostaglandin A1 CAS 14152-28-4View Details
Prostaglandin A1 CAS 14152-28-4View Details
14152-28-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1