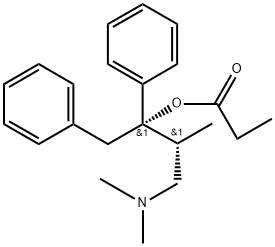
PROPOXYPHENE
- CAS NO.:469-62-5
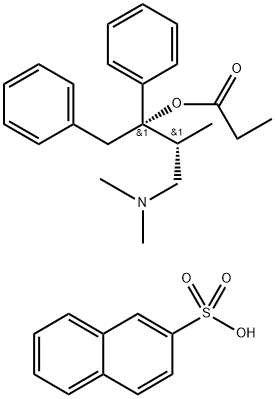
- Empirical Formula: C22H29NO2
- Molecular Weight: 339.48
- MDL number: MFCD00242869
- EINECS: 207-420-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
What is PROPOXYPHENE?
Toxicity
Coma, respiratory depression, circulatory collapse, and pulmonary edema. Seizures occur more frequently in patients with propoxyphene intoxication than in those with opiate intoxication. LD50=230mg/kg (orally in rat, Emerson)
The Uses of PROPOXYPHENE
Analgesic.
Background
Dextropropoxyphene is an opioid analgesic manufactured by Eli Lilly and Company. It is used in the symptomatic treatment of mild pain. It displays antitussive and local anaesthetic actions. Due to the risk of cardiac arrhythmias and overdose, possibly leading to death, dextropropoxyphene has been withdrawn from the market in Europe and the United States. The drug is often referred to as the general form, "propoxyphene", however only the dextro-isomer (dextropropoxyphene) has any analgesic effect. The levo-isomer appears to exhibit a very limited antitussive effect.
Indications
For the relief of mild to moderate pain.
Definition
ChEBI: The (1S,2R)-(+)-diastereoisomer of propoxyphene.
brand name
Darvon-N (Xanodyne).
Biological Functions
Propoxyphene (dextropropoxyphene; Darvon) is structurally
related to methadone but is much less potent as
an analgesic. Compared with codeine, propoxyphene is
approximately half as potent and is indicated for the
treatment of mild pain. It is not antipyretic or antiinflammatory
like aspirin and is less useful than aspirin in
most cases of mild pain. Toxicity from propoxyphene,
especially in combination with other sedatives, such as
alcohol, has led to a decrease in its use. Death following
ingestion of alcohol in combination with propoxyphene
can occur rapidly (within 20 minutes to 1 hour). The
drug is not indicated for those with histories of suicide
or depressive illnesses.
Like meperidine, propoxyphene has an active
metabolite, norpropoxyphene, that is not analgesic but
has excitatory and local anesthetic effects on the heart
similar to those of quinidine. Use of the drug during
pregnancy is not safe.Teratogenic effects have been observed
in newborns, as have withdrawal signs at birth.
As with morphine, propoxyphene requires adequate hepatic
and renal clearance to prevent toxicity and drug
accumulation. It is thus contraindicated in the elderly
patient and those with renal or liver disease.
Propoxyphene interacts with several drugs. The use
of sedatives in combination with propoxyphene can be
fatal. In addition, the metabolism of the drug is increased
in smokers due to induction of liver enzymes.
Thus, smokers may require a higher dose of the drug for
pain relief. Propoxyphene enhances the effects of both
warfarin and carbamazepine and may increase the toxicity
associated with both drugs, such as bleeding and sedation,
respectively.
The abuse liability of propoxyphene is low because
of the extreme irritation it causes at the site of injection.
Oral use is the preferred route of administration for this
reason.
General Description
Most of the structural changes to the methadone skeletonresulted in compounds with decreased opioid potencies, thusmost of these compounds, with the exception of LAAM werenot developed. Propoxyphene is a derivative of methadonemarketed in 1957 as the enantiomerically pure (2S, 3R)-4-(Dimethylamino)-3-methyl-1,2,-diphenyl-2-butanol propionate(ester). It is only about 1/10th as potent as morphine asan analgesic yet retains all the same opioid adverse effects.One propoxyphene 65-mg capsule has the same analgesic effectof 650 mg of aspirin or 1,000 mg of acetaminophen, thusoverdoses of propoxyphene can occur if patients do not followthe prescribed dose. Between 1981 and 1999, 2,110 accidentaldeaths were reported because of propoxyphene.Propoxyphene and all propoxyphene combination productsare listed using the Beers criteria as medications to avoid inpatients older than 65 years of age. The metabolism ofpropoxyphene also contributes to the potential dangers of thedrug. Propoxyphene is metabolized via N-demethylation toform norpropoxyphene. Norpropoxyphene has been shownto build up in cardiac tissues and result in naloxone-insensitivecardiotoxicity. The weak analgesic action and potentialrisk to the patient have some health practitioners advocatingto remove all drugs containing propoxyphene from the market.The hydrochloride salt is marketed as Darvon, the napsylatesalt as Darvon-N, both salts are also available combinedwith acetaminophen (Darvocet, Darvocet-N) and apropoxyphene, aspirin, caffeine product is also available.
Pharmacokinetics
Propoxyphene, a synthetic opiate agonist, is structurally similar to methadone. Its general pharmacologic properties are those of the opiates as a group. The analgesic effect of propoxyphene is due to the d-isomer, dextropropoxyphene. It binds to the opiate receptors and leads to a decrease of the perception of pain stimuli. Propoxyphene possesses little to no antitussive activity and no antipyretic action.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Human systemic effects by ingestion: change in cardiac rate, respiratory depression, and coma. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Hepatic
Properties of PROPOXYPHENE
| Melting point: | 75-76° |
| Boiling point: | 475.43°C (rough estimate) |
| alpha | D25 +67.3° (c = 0.6 in chloroform) |
| Density | 1.0751 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| pka | pKa 6.3(50% aq EtOH) (Uncertain) |
| EPA Substance Registry System | Propoxyphene (469-62-5) |
Safety information for PROPOXYPHENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P337+P313:IF eye irritation persists: Get medical advice/attention. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for PROPOXYPHENE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4