PROPERICYAZINE
- CAS NO.:2622-26-6
- Empirical Formula: C21H23N3OS
- Molecular Weight: 365.49
- MDL number: MFCD00210348
- EINECS: 220-071-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
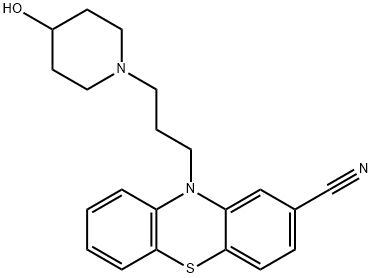
What is PROPERICYAZINE?
Toxicity
In milder cases of phenothiazine overdosage the patient may be agitated, delirious and confused. Frequently he is lethargic or in a comatose state. Twitching, dystonic movements or convulsions may be present and hypotension, cardiovascular collapse, arrhythmias and hypothermia might be observed.
Chemical properties
Yellow Solid
Originator
Aolept, Bayer Vital
The Uses of PROPERICYAZINE
Psychotherapeutic phenothiazine. Antipsychotic.
The Uses of PROPERICYAZINE
Spectrophotometric reagent for palladium and ruthenium.
Background
Periciazine is a phenothiazine of the piperidine group. It has been shown to reduce pathologic arousal and affective tension in some psychotic patients, while the symptoms of abnormal mental integration are relatively unaffected. It is a sedative phenothiazine with weak antipsychotic properties. It also has adrenolytic, anticholinergic, metabolic and endocrine effects and an action on the extrapyramidal system. It is used as an adjunctive medication in some psychotic patients, for the control of residual prevailing hostility, impulsiveness and aggressiveness. Pericyazine, like other phenothiazines, is presumed to act principally in the subcortical areas, by producing what has been described as a central adrenergic blockade.
Indications
For use as adjunctive medication in some psychotic patients. Propericiazine (Pericyazine)is used for the control of residual prevailing hostility, impulsiveness and aggressiveness.
Definition
ChEBI: A member of the class of phenothiazines that is 10H-phenothiazine substituted by a 3-(4-hydroxypiperidin-1-yl)propyl group at the nitrogen atom and a carbonitrile group at position 2. Periciazine is a first generation antipsychotic.
Manufacturing Process
2-Cyano-10-(3-methanesulfonyloxypropyl)phenthiazine and 4hydroxypiperidine in toluene were heated under reflux with stirring. The reaction mixture was allowed to cool and water was added. The resulting toluene solution layer was decanted and washed twice with water. The toluene solution was then stirred with 5% hydrochloric acid. The hydrochloride of the desired phenthiazine base precipitated in gummy condition in the aqueous layer. This was decanted and treated with sodium hydroxide (density 1.33). It was then extracted three times with ethyl acetate. The extracts were dried over sodium sulfate, filtered and concentrated in vacuum. A resinous product was obtained. This product was dissolved in a mixture of benzene and cyclohexane and chromatographed on a column containing alumina. The chromatographed product was eluted successively with mixtures of benzene and cyclohexane and then with benzene and finally with a mixture of benzene and ethyl acetate. The eluates were evaporated to yield a crude product. This product was recrystallised from aqueous ethanol (40% water) and yielded 2cyano-10-[3-(4-hydroxy-1-piperidyl)propyl]phenthiazine as white crystals.
Therapeutic Function
Neuroleptic
Pharmacokinetics
Pericyazine is a phenothiazine of the piperidine group. It has been shown to reduce pathologic arousal and affective tension in some psychotic patients, while the symptoms of abnormal mental integration are relatively unaffected. It is a sedative phenothiazine with weak antipsychotic properties. It also has adrenolytic, anticholinergic, metabolic and endocrine effects, and an action on the extrapyramidal system.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Used as an antipsychotic agent. When heated to decomposition it emits very toxic fumes of CN-, NOx, and SOx. See also NITRILES.
Metabolism
Not Available
Purification Methods
It recrystallises from a saturated solution in cyclohexane. It is antipsychotic and is a sensitive reagent for Pd, Va, Ru, Rh and Au. [Gowda et al. Anal Chem 55 1816 1983, Anal Chim Acta 154 347 1983, Beilstein 27 III/IV 4110.]
Properties of PROPERICYAZINE
| Melting point: | 116-117° |
| Boiling point: | 593.4±50.0 °C(Predicted) |
| Density | 1.1198 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.87±0.20(Predicted) |
| color | Light Yellow to Yellow |
| Water Solubility | 38.01mg/L(37 ºC) |
| CAS DataBase Reference | 2622-26-6 |
Safety information for PROPERICYAZINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for PROPERICYAZINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1