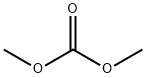
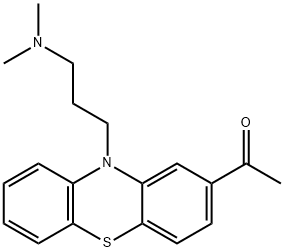
Promazine
- CAS NO.:58-40-2
- Empirical Formula: C17H20N2S
- Molecular Weight: 284.4191
- MDL number: MFCD00242576
- EINECS: 2003820
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:35
What is Promazine?
Absorption
Absorption may be erratic and peak plasma concentrations show large interindividual differences.
Toxicity
Side effects include: extrapyramidal symptoms, drowsiness, weight gain, dry mouth, constipation, endocrine effects (such as gynaecomastia and menstrual disturbance), sensitivity to sunlight and haemolytic anaemia.
The Uses of Promazine
In psychiatric practice, promazine is used in minor cases of psychomotor excitement in schizophrenics, in paranoid and manic-depressive conditions, for neurosis, alcoholic psychosis, and others. It is sometimes used in anesthesiological practice.
The Uses of Promazine
ntipsychotic.
The Uses of Promazine
Promazine is an antipsychotic and a tranquilizer.
Indications
Used as an adjunct for short term treatment of moderate and severe psychomotor agitation. Also used to treat agitation or restlessness in the elderly.
Background
A phenothiazine with actions similar to chlorpromazine but with less antipsychotic activity. It is primarily used in short-term treatment of disturbed behavior and as an antiemetic. It is currently not approved for use in the United States.
Definition
ChEBI: A phenothiazine deriative in which the phenothiazine tricycle has a 3-(dimethylaminopropyl) group at the N-10 position.
brand name
Sparine (Baxter Healthcare); Sparine (Wyeth).
General Description
Promazine, 10-[3-(dimethylamino) propyl-(phenothiazine monohydrochloride (Sparine), was introducedinto antipsychotic therapy after its 2-chloro-substitutedrelative. The 2H-substituent vis-à-vis the 2Clsubstituent gives a milligram potency decrease as an antipsychotic,as encompassed in Gordon’s rule. Tendency toEPS is also lessened, which may be significant, especially ifit is decreased less than antipsychotic potency.
Pharmacokinetics
Promazine belongs to a group of medications known as the phenothiazine antipsychotics. It acts by blocking a variety of receptors in the brain, particularly dopamine receptors. Dopamine is involved in transmitting signals between brain cells. When there is an excess amount of dopamine in the brain it causes over-stimulation of dopamine receptors. These receptors normally act to modify behaviour and over-stimulation may result in psychotic illness. Promazine hydrochloride blocks these receptors and stops them becoming over-stimulated, thereby helping to control psychotic illness. Promazine has weak extrapyramidal and autonomic side effects which lead to its use in the elderly, for restless or psychotic patients. Its anti-psychotic effect is also weaker and it is not useful in general psychiatry.
Synthesis
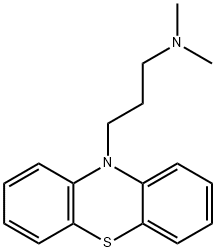
Promazine, 10-(3-dimethylaminopropyl)phenothiazine (6.1.1), is prepared by the alkylation of phenothiazine with 3-dimethylaminopropylchloride in the presence of sodium amide [1¨C3].
Metabolism
Hepatic, primarily to N-desmethylpromazine and promazine sulfoxide.
Properties of Promazine
| Melting point: | 25°C |
| Boiling point: | bp0.3 203-210° |
| Density | 1.1256 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | pKa 9.4(H2O,t =24±1) (Uncertain) |
| form | Solid |
| color | Off-White to Pale Beige |
| Water Solubility | 14.22mg/L(24 ºC) |
| Stability: | Hygroscopic |
| NIST Chemistry Reference | Promazine(58-40-2) |
Safety information for Promazine
Computed Descriptors for Promazine
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran


You may like
-
 58-40-2 Chlorpromazine EP Impurity C 99%View Details
58-40-2 Chlorpromazine EP Impurity C 99%View Details
58-40-2 -
 58-40-2 98.10View Details
58-40-2 98.10View Details
58-40-2 -
 Ethoxymethylenemalononitrile 99% HPLCView Details
Ethoxymethylenemalononitrile 99% HPLCView Details
123-06-8 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 401-95-6 99% HPLCView Details
401-95-6 99% HPLCView Details
401-95-6 -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1