Hydroxyacetone
Synonym(s):1-Hydroxy-2-propanone;Acetol
- CAS NO.:116-09-6
- Empirical Formula: C3H6O2
- Molecular Weight: 74.08
- MDL number: MFCD00004669
- EINECS: 204-124-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
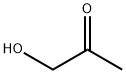
What is Hydroxyacetone?
Chemical properties
colourless to yellow liquid
The Uses of Hydroxyacetone
Hydroxyacetone is used as a reagent in organic chemical reactions. It also serves as a component for Mannich reaction and aldol reactions. It is also used in the syntheses of 2-oxo-propionaldehyde, imidazoles, polyols, acrolein, dyes and skin tanning agents. It yields (R)-1,2-propanediol upon reduction of hydroxyacetone in the presence of a microbial cell catalyst.
The Uses of Hydroxyacetone
Hydroxyacetone is a chemical reagent used in various organic chemical reactions. It is a component of the Mannich reaction, amino acid caalyzes direct asymmetric aldol reactions. In the pharmaceutical setting, this compound is used in the synthesis of imidazoles acting as potent and orally active antihypertensive agents.
The Uses of Hydroxyacetone
Reagent in organic synthesis; protecting group for the synthesis of peptides.
What are the applications of Application
Hydroxyacetone is used to study aerobic oxidation of glycerol and propanediol over metal oxide supported gold nanoparticles in methanol.
Definition
ChEBI: A propanone that is acetone in which one of the methyl hydrogens is replaced by a hydroxy group.
General Description
Hydroxyacetone (Acetol) is important for the manufacture of polyols, acrolein, dyes and skin tanning agents. It undergoes asymmetric reduction to yield (R)-1,2-propanediol in the presence of microbial cell catalyst.
Safety Profile
Moderately toxic by ingestion. Mutation data reported. An allergen. Implicated in aplastic anemia. A 10 gram dose may be fatal to an adult. skin contact, inhalation, or ingestion can cause asthma, sneezing, irritation of eyes and nose, hives, and eczema. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes.
Synthesis
Hydroxyacetone is produced from Bromoacetone plus sodium formate, followed by Methanol hydrolysis of the formed ester. Or by biochemical synthesis from Propylene glycol with sorbose bacterium.
Properties of Hydroxyacetone
| Melting point: | -17 °C (lit.) |
| Boiling point: | 145-146 °C (lit.) |
| Density | 1.082 g/mL at 25 °C (lit.) |
| vapor pressure | 9.01hPa at 25℃ |
| FEMA | 4462 | HYDROXYACETONE |
| refractive index | n |
| Flash point: | 133 °F |
| storage temp. | 2-8°C |
| solubility | water: miscible |
| form | clear liquid |
| pka | 13.14±0.10(Predicted) |
| Specific Gravity | 1.090 (20/4℃) |
| color | Colorless liquid |
| Odor | at 10.00 % in dipropylene glycol. pungent sweet caramellic ethereal |
| Water Solubility | Miscible with water, alcohol and ether. |
| Sensitive | Hygroscopic |
| Merck | 14,65 |
| JECFA Number | 1945 |
| BRN | 605368 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture - hygroscopic. |
| CAS DataBase Reference | 116-09-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanone, 1-hydroxy-(116-09-6) |
| EPA Substance Registry System | 1-Hydroxyacetone (116-09-6) |
Safety information for Hydroxyacetone
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for Hydroxyacetone
| InChIKey | XLSMFKSTNGKWQX-UHFFFAOYSA-N |
Hydroxyacetone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Hydroxyacetone CAS 116-09-6View Details
Hydroxyacetone CAS 116-09-6View Details
116-09-6 -
 Hydroxyacetone 95% CAS 116-09-6View Details
Hydroxyacetone 95% CAS 116-09-6View Details
116-09-6 -
 Hydroxyacetone (stabilized with Na2CO3) CAS 116-09-6View Details
Hydroxyacetone (stabilized with Na2CO3) CAS 116-09-6View Details
116-09-6 -
 ACETONE ALCOHOL DECOLOURIZER For Microscopy CAS 116-09-6View Details
ACETONE ALCOHOL DECOLOURIZER For Microscopy CAS 116-09-6View Details
116-09-6 -
 Hydroxyacetone CAS 116-09-6View Details
Hydroxyacetone CAS 116-09-6View Details
116-09-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
