Probucol
Synonym(s):Probucol
- CAS NO.:23288-49-5
- Empirical Formula: C31H48O2S2
- Molecular Weight: 516.84
- MDL number: MFCD00079281
- EINECS: 245-560-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
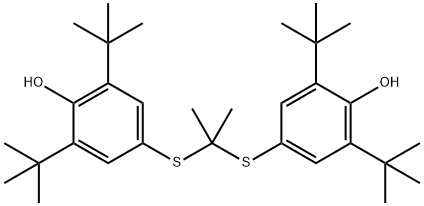
What is Probucol?
Absorption
Absorption from the gastrointestinal tract is limited and variable (about 7%).
Chemical properties
White Solid
The Uses of Probucol
Probucol is an antilipemic.
The Uses of Probucol
antihyperlipidemic
The Uses of Probucol
anti-hyperlipoproteinemic
The Uses of Probucol
An antioxidant, anti-inflammatory, and hypocholesterolemic agent which inhibits atherogenesis in murine models.
Background
A drug used to lower LDL and HDL cholesterol yet has little effect on serum-triglyceride or VLDL cholesterol. (From Martindale, The Extra Pharmacopoeia, 30th ed, p993).
Indications
Used to lower LDL and HDL cholesterol.
What are the applications of Application
Probucol is an antioxidant, anti-inflammatory, and hypocholesterolemic agent which inhibits atherogenesis in murine models
Definition
ChEBI: A dithioketal that is propane-2,2-dithiol in which the hydrogens attached to both sulfur atoms are replaced by 3,5-di-tert-butyl-4-hydroxyphenyl groups. An anticholesteremic drug with antioxidant and anti-inflammatory properties, it is used to treat high l vels of cholesterol in blood.
brand name
Lorelco (Sanofi Aventis).
Biological Functions
Probucol (Lorelco) is a hypocholesterolemic drug with
few side effects that modestly (15–30%) decreases elevated
plasma LDL cholesterol levels. The marginal LDL-lowering action plus reports that it can lower
HDL cholesterol resulted in its discontinuation as a
hypocholesterolemic drug. However, it still may reduce
the risk of CHD because it is a powerful antioxidant.
The oxidation hypothesis of atherosclerosis states
that oxidation of lipids in LDL is required for LDL uptake
by macrophages and smooth muscle cells in the intima
of arteries, leading to their transformation to foam cells, an early event in atherogenesis. A recent clinical
trial reported that use of probucol decreased the rate of
restenosis of coronary arteries by 50% in patients who
underwent angioplasty. Fluvastatin also has potent antioxidant
properties that may contribute to its antiatherosclerotic
effects.These findings suggest that reducing
high plasma lipids may not be the only approach to retarding
the progression of atherosclerosis and decreasing
the risk of coronary heart disease.
General Description
Probucol, 4,4' -[(1-methylethylidene)bis(thio)]bis[2,6-bis(1,1-dimethylethyl)phenol], DH-581(Lorelco), is a chemical agent that was developed for the plasticsand rubber industry in the 1960s. The probucol moleculehas two tertiary butylphenol groups linked by a dithiopropylidenebridge, giving it a high lipophilic character with strongantioxidant properties. In humans, it causes reduction of bothliver and serum cholesterol levels, but it does not alter plasmatriglycerides. It reduces LDL and (to a lesser extent) HDLlevels by a unique mechanism that is still not clearly delineated.The reduction of HDL may be caused by the ability ofprobucol to inhibit the synthesis of apoprotein A-1, a majorprotein component of HDL. It is effective at reducing levelsof LDL and is used in hyperlipoproteinemias characterized byelevated LDL levels.
Biological Activity
Antioxidant, anti-inflammatory and hypocholesterolemic agent. Inhibits atherogenesis in genetically hypercholesterolemic rabbits (Watanabe) and attenuates ischemia/reperfusion-induced cardiomyocyte apoptosis.
Mechanism of action
Probucol reduces the overall level of cholesterol—primarily low-density lipoproteins— without having an effect on triglycerides and very low-density lipoproteins. It has been suggested that it inhibits synthesis of cholesterol itself and increases removal of bile salts. Upon using this drug, a fraction of low-density proteins is reduced; however, even more significant is the reduction of high-density proteins. From the epidemiological point of view, this is dangerous, because lowering the concentration of high-density proteins means less cholesterol is removed from tissues. However, in any case, probucol lowers the level of cholesterol in the plasma by 10–15%. Moreover, it has been shown that probucol facilitates reduction of necrotic zones in myocardial ischemia.
Pharmacokinetics
Probucol lowers cholesterol levels by increasing LDL (low-density lipoprotein) breakdown. Additionally, probucol may inhibit cholesterol synthesis and delay cholesterol absorption. Probucol is a powerful antioxidant drug normally used to prevent vascular disease caused by the free radicals in the body.
Pharmacology
Being a lipophilic compound, it is easily distributed into fatty tissue and, as a result, approximately 20% of its maximum concentration in the blood is still maintained for 6 months.
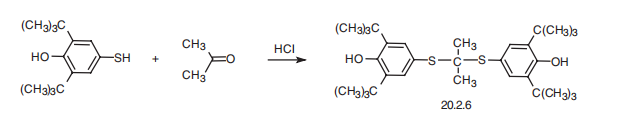
Synthesis
Probucol, bis(3,5-tert-butyl-4-hydroxyphenyl)mercaptol acetone (20.2.6), is synthesized by thioketalizing acetone with 2,6-di-tert-butyl-4-mercaptophenol in the presence of hydrogen chloride.
Metabolism
Not Available
Properties of Probucol
| Melting point: | 126-128°C |
| Boiling point: | 571.58°C (rough estimate) |
| Density | 1.0008 (rough estimate) |
| refractive index | 1.5341 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | neat |
| pka | 10.27±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Merck | 14,7755 |
| CAS DataBase Reference | 23288-49-5(CAS DataBase Reference) |
Safety information for Probucol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Probucol
| InChIKey | FYPMFJGVHOHGLL-UHFFFAOYSA-N |
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
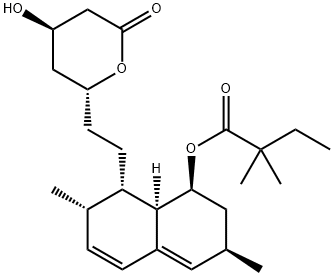
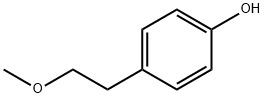
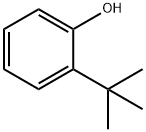
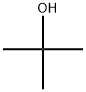
You may like
-
 Probucol 98% CAS 23288-49-5View Details
Probucol 98% CAS 23288-49-5View Details
23288-49-5 -
 Probucol CAS 23288-49-5View Details
Probucol CAS 23288-49-5View Details
23288-49-5 -
 Probucol CAS 23288-49-5View Details
Probucol CAS 23288-49-5View Details
23288-49-5 -
 Probucol CAS 23288-49-5View Details
Probucol CAS 23288-49-5View Details
23288-49-5 -
 Probucol CAS 23288-49-5View Details
Probucol CAS 23288-49-5View Details
23288-49-5 -
 Probucol Related Compound A CAS 23288-49-5View Details
Probucol Related Compound A CAS 23288-49-5View Details
23288-49-5 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3
