tert-Butanol
Synonym(s):1-Butanol;TBA;tert-Butanol;Butyl alcohol;2-Methyl-2-propanol
- CAS NO.:75-65-0
- Empirical Formula: C4H10O
- Molecular Weight: 74.12
- MDL number: MFCD00004464
- EINECS: 200-889-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:51
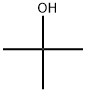
What is tert-Butanol?
Description
t-Butanol exists in a lab environment as either a liquid or solid, depending on the ambient temperature. It finds use generally as a solvent for certain reactions, and occasionally as a reagent, as in Curtius rearrangements, for example.
Chemical properties
tert-Butanol ((CH?)?COH) is a white crystalline solid or colorless liquid (above 25°C) with a camphor-like odor, soluble in water and miscible with alcohols, ethers, and other organic solvents. It is highly flammable and easily ignited by heat, sparks, or flames, with vapors forming explosive mixtures in air. Contact with oxidizing agents, strong mineral acids, or hydrochloric acid may cause fires or explosions.
As a polar organic solvent, it is used as a dehydrating agent, solvent, and denaturant for ethyl alcohol, and is extensively employed in the production of flavors, perfumes, paint removers, methyl methacrylate plastics, and flotation devices. It is a metabolite of the gasoline additive MTBE. In cosmetic and food applications, it is utilized to create artificial musk, fruit essences, perfumes, and coatings for metal and paperboard food containers, as well as in industrial cleaners and pharmaceutical extraction processes.
Physical properties
tert-Butanol is a colorless liquid or crystalline solid with a camphor-like odor. An odor threshold concentration of 2,900 mg/m3 (957 ppmv) was experimentally determined by Dravnieks (1974), while a later study by Nagata and Takeuchi (1990) reported a threshold of 220 ppbv.
The Uses of tert-Butanol
DPPA (642 mg, 2.34 mmol) and TEA (236 mg, 2.34 mmol) were added to a solution of the SM (300 mg, 2.34 mmol) in t-BuOH (10 mL) at 50 C. The reaction mixture was stirred at 90 C for 16 h. After concentration, the residue was purified by silica gel column chromatography (1:4 PE/EtOAc) to provide the product as a yellow solid. [412 mg, 66%]
What are the applications of Application
tert-Butanol is a solvent and isomer of butanol
Definition
Tert-butanol is a tertiary alcohol alcohol that is isobutane substituted by a hydroxy group at position 2. It has a role as a human xenobiotic metabolite. It derives from a hydride of an isobutane.
Production Methods
tert-Butanol is produced as a by-product from the isobutane oxidation process for manufacturing propylene oxide. It is also produced by the acidcatalyzed hydration of isobutylene, a process no longer used in the United States.
General Description
Colorless oily liquid with a sharp alcohol odor. Floats and mixes with water. Produces irritating vapor. Freezing point is 78°F.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Alcohols can undergo violent or explosive reactions with various substances: acetyl bromide reacts violently with alcohols or water; mixtures with concentrated sulfuric acid and strong hydrogen peroxide may cause explosions (e.g., dimethylbenzylcarbinol with 90% H?O? acidified with H?SO?); ethanol forms powerful explosives with concentrated H?O?; and hydrogen peroxide with alcohols like 1-phenyl-2-methylpropyl alcohol can explode when acidified. Alkyl hypochlorites, obtained from hypochlorous acid and alcohols, are highly explosive, decomposing in cold and exploding upon heat or sunlight exposure, with tertiary hypochlorites being more stable than primary or secondary. Base-catalyzed reactions of isocyanates with alcohols require inert solvents to prevent explosive violence. Additionally, alcohols can attack plastics.
Hazard
Irritant to eyes and skin. Flammable, dan- gerous fire risk. Central nervous system impair- ment. Questionable carcinogen.
Health Hazard
tert-Butanol is more toxic than secbutylalcohol but less toxic than the primaryalcohol. However, its narcotic actionis stronger than that of n-butanol. Inhalationmay cause drowsiness and mild irritationof the skin and eyes. Ingestion may produceheadache, dizziness, and dry skin.Acute oral LD50 value (rats): 3500 mg/kg.
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by ingestion, intravenous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Dangerous fire hazard when exposed to heat or flame. Moderately explosive in the form of vapor when exposed to flame. Ignites on contact with potassium-sodum alloys. To fight fire, use alcohol foam, CO2, dry chemical. Incompatible with oxidizing materials, H202. See also n-BUTYL ALCOHOL and ALCOHOLS.
Potential Exposure
tert-Butanol is used as solvents for paints, lacquers, varnishes, natural and synthetic resins, gums, vegetable oils, dyes, camphor, and alkaloids. They are also used as an intermediate in the manufacture of pharmaceuticals and chemicals; In the manufacture of artificial leather, safety glass; rubber and plastic cements, shellac, raincoats, photographic films, perfumes; and in plastic fabrication.
Carcinogenicity
There were increased incidences of renal tubule adenoma and carcinoma in male rats, transitional epithelia hyperplasia of the kidney in male and female rats, follicular cell adenoma of the thyroid in female mice, and follicular cell hyperplasia of the thyroid and inflammation and hyperplasia of the urinary bladder in male and female mice. In addition, a slight increase in follicular cell adenoma or carcinoma of the thyroid (combined) in male mice may have been related to exposure to t-butyl alcohol. t-Butyl alcohol was inactive on mouse skin as a complete carcinogen or as a tumor promoter.
Source
Detected in a distilled water-soluble fraction of 94 octane unleaded gasoline at a concentration of 3.72 mg/L (Potter, 1996)
Shipping
UN1120 Butanols, Hazard Class: 3; Labels: 3— Flammable liquid. UN1212 Isobutanol or Isobutyl alcohol, Hazard Class: 3; Labels: 3—Flammable liquid
Purification Methods
Tert-butanol is commercially produced by hydrating 2-methylpropene in dilute sulfuric acid. It can be dried using desiccants such as CaO, K?CO?, CaSO?, or MgSO?, followed by filtration and fractional distillation. Further drying can be achieved by refluxing with and distilling from magnesium activated with iodine, or with small amounts of calcium, sodium, or potassium under nitrogen. Alternative methods include passage through a 4? molecular sieve column, refluxing with tert-butyl phthalate or succinate, treatment with excess aluminum tert-butoxide, or standing with CaH? followed by distillation. For highly aqueous samples, benzene can be added to remove water via azeotropic distillation (b.p. 67.3?°C). Trace isobutylene can be eliminated by bubbling dry nitrogen through the alcohol at 40–50?°C. Purification may also involve fractional crystallization via partial freezing (while excluding moisture) or distillation from CaH? into activated 4? molecular sieves. For rapid purification, the alcohol is dried over CaH? (5% w/v), distilled, and stored over 3? molecular sieves.
Incompatibilities
tert-Butanol is incompatible with strong acids (including mineral acid), including mineral acids; strong oxidizers or caustics, aliphatic amines; isocyanates, alkali metals (i.e., lithium, sodium, potassium, rubidium, cesium, francium).
Waste Disposal
Incineration, or bury absorbed waste in an approved land fill.
Properties of tert-Butanol
| Melting point: | 23-26 °C (lit.) |
| Boiling point: | 83 °C (lit.) |
| Density | 0.775 g/mL at 25 °C (lit.) |
| vapor density | 2.55 (vs air) |
| vapor pressure | 31 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 95 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: miscible |
| form | Liquid After Melting |
| appearance | Colorless liquid |
| pka | 19(at 25℃) |
| color | APHA: ≤20 |
| Odor | Characteristic; camphor-like; pungent. |
| Relative polarity | 0.389 |
| PH Range | 7 |
| Odor Threshold | 4.5ppm |
| explosive limit | 2.3-8.0%(V) |
| Water Solubility | soluble |
| λmax | λ: 215 nm Amax: 1.00 λ: 230 nm Amax: 0.50 λ: 250 nm Amax: 0.20 λ: 300-350 nm Amax: 0.01 |
| JECFA Number | 85 |
| Merck | 14,1542 |
| BRN | 906698 |
| Henry's Law Constant | 1.22 at 25 °C (static headspace-GC, Merk and Riederer, 1997) |
| Exposure limits | TLV-TWA 300 mg/m3 (100 ppm) (ACGIH);
IDLH 8000 ppm. |
| Dielectric constant | 7.8(19℃) |
| Stability: | Stable. Very flammable. Incompatible with strong oxidizing agents, copper, copper alloys, alkali metals, aluminium. |
| CAS DataBase Reference | 75-65-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanol, 1,1-dimethyl-(75-65-0) |
| EPA Substance Registry System | tert-Butanol (75-65-0) |
Safety information for tert-Butanol
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for tert-Butanol
| InChIKey | DKGAVHZHDRPRBM-UHFFFAOYSA-N |
tert-Butanol manufacturer
Jaiswal S Cyber Shop
Antares Chem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Tertiary Butanol 99%View Details
Tertiary Butanol 99%View Details -
 TERTIARY BUTANOL 99%View Details
TERTIARY BUTANOL 99%View Details -
 Tert-Butanol 99%View Details
Tert-Butanol 99%View Details -
 Tert- Butanol 99%View Details
Tert- Butanol 99%View Details -
 Tertiary Butanol CASView Details
Tertiary Butanol CASView Details -
 Butanol CASView Details
Butanol CASView Details -
 Tertiary Butanol CASView Details
Tertiary Butanol CASView Details -
 Butanol, >99%, 200 litre drum, for paint and thinner industryView Details
Butanol, >99%, 200 litre drum, for paint and thinner industryView Details
71-36-3
