Ezetimibe
Synonym(s):(3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one;Ezetimibe
- CAS NO.:163222-33-1
- Empirical Formula: C24H21F2NO3
- Molecular Weight: 409.43
- MDL number: MFCD00937872
- EINECS: 682-606-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
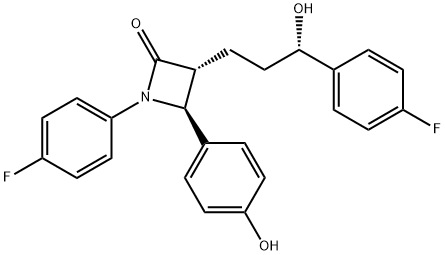
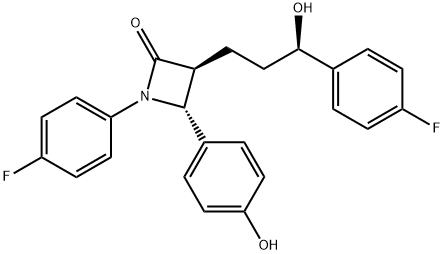
What is Ezetimibe?
Absorption
Administration of a single 10-mg dose of ezetimibe in fasted adults resulted in peak plasma concentrations (Cmax) of 3.4-5.5 ng/mL within 4-12 hours (Tmax). The Cmax of the major pharmacologically-active metabolite, ezetimibe-glucuronide, was 45-71 ng/mL and its Tmax was 1-2 hours. Food consumption has minimal effect on ezetimibe absorption, but the Cmax is increased by 38% when administered alongside a high-fat meal. The true bioavailability of ezetimibe cannot be determined, as it is insoluble in aqueous media suitable for intravenous injection.
Toxicity
Oral LD50 and intraperitoneal LD50 in rat were >2000 mg/kg. Estimated oral LD50 values in mouse and dog are >5000 mg/kg and >3000 mg/kg, respectively. One case of accidental overdose occurred in clinical studies in one female patient with homozygous sitosterolemia receiving 120 mg/day for 28 days with no reported clinical or laboratory adverse events. In case of overdose, symptomatic treatment is recommended.
Description
Ezetimibe is a once-daily orally active cholesterol absorption inhibitor, launched as a hypolipidemic agent. The one-step diastereo- and enantioselective formation of β-lactams starting from commercially available (3S)-hydroxy-y-lactone is the key point of the asymmetric synthesis of ezetimibe. The 2-azetidinone class was initially designed as acylcoenzyme A: cholesterol acyltransferase (ACAT) inhibitors but experimental data suggest that this compound acts in the intestinal wall to inhibit cholesterol through a novel mechanism with an as yet undiscovered target. Orally administered ezetimibe inhibited increases in plasma cholesterol in four cholesterol-fed animals species (hamster, rats, dogs and rhesus monkeys). In rats cannulated in the intestine and bile duct, [3H]-ezetimibe inhibited cholesterol absorption by more than 95%. In cholesterol-fed LDL receptor+apoE knockout mice, treatment with ezetimibe reduced atherosclerotic lesion cross sectional area by 48% in the aorta and 20% in the carotid artery. Moreover, the plasma cholesterol levels were reduced and the progression of lesions was inhibited. Ezetimibe is highly protein bound and is metabolized by the liver to its glucuronide metabolite, which represents 80-90% of circulating ezetimibe. About 90% of ezetimibe and/or the glucuronide metabolite are excreted in the feces and 10% in the urine. The parent compound and its glucuronide metabolite undergo enterohepatic recirculation; in consequence, the drug is slowly eliminated. In hypercholesterolemic patients, ezetimibe (10 mglday, 12 weeks) reduced LDL cholesterol by 18% and total cholesterol by 12%, with a similar safety profile to placebo. Co-administration of ezetimibe with statins or fenofibrate lowered LDL cholesterol levels more than either monotherapy. Ezetimibe was well tolerated and interaction studies provided evidence that ezetimibe had no significant effect on the activity of major CYP450 drug-metabolizing enzymes. Moreover, no pharmacokinetic/pharmacodynamic interactions were seen between ezetimibe and statins and others frequently administered drugs. .
Chemical properties
White Solid
Originator
Schering-Plough (USA)
The Uses of Ezetimibe
Ezetimibe (9) was approved as the first hypolipidemic drug to act by blocking the absorption of dietary cholesterol. This drug was discovered by Schering-Plough and is codeveloped and co-marketed by Merck and Schering-Plough for the treatment of hypercholesterolemia and also two less common forms of hyperlipidemia: homozygous familial hypercholesterolemia and homozygous sitosterolemia.
The Uses of Ezetimibe
antibacterial
The Uses of Ezetimibe
A cholesterol transport inhibitor that binds to NPC1L1
The Uses of Ezetimibe
For use as adjunctive therapy to diet for the reduction of elevated total-C, LDL-C, and Apo B in patients with primary (heterozygous familial and non-familial) hypercholesterolemia.
Indications
Ezetimibe is indicated to reduce elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with primary hyperlipidemia, alone or in combination with an HMG-CoA reductase inhibitor (statin). It is also indicated to reduce elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with mixed hyperlipidemia in combination with fenofibrate, and to reduce elevated total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH), in combination with atorvastatin or simvastatin. Ezetimibe may also be used to reduce elevated sitosterol and campesterol in patients with homozygous sitosterolemia (phytosterolemia).
Background
Ezetimibe is a lipid-lowering compound that inhibits intestinal cholesterol and phytosterol absorption. The discovery and research of this drug began in the early 1990s, after the intravenous administration of radiolabelled ezetimibe in rats revealed that it was being localized within enterocytes of the intestinal villi - this prompted studies investigating the effect of ezetimibe on intestinal cholesterol absorption. Ezetimibe is used as an adjunctive therapy to a healthy diet to lower cholesterol levels in primary hyperlipidemia, mixed hyperlipidemia, homozygous familial hypercholesterolemia (HoFH), and homozygous sitosterolemia (phytosterolemia).
brand name
Zetia (Merck/Schering-Pough);Ezetrol.
Biological Functions
Ezetimibe lowers plasma cholesterol levels by inhibiting the absorption of cholesterol at the brush border of the small intestine. Specifically, it has been proposed to bind to a specific transport protein located in the wall of the small intestine, resulting in a reduction of cholesterol transport and absorption. Ezetimibe appears to be selective in its actions in that it does not interfere with the absorption of triglycerides, lipid-soluble vitamins or other nutrients. The decreased absorption of cholesterol eventually leads to enhanced receptor-mediated LDL uptake similar to that seen with bile acid sequestrants and HMGRIs. When used as monotherapy, the decreased absorption of cholesterol causes a compensatory increase in cholesterol biosynthesis. This is similar to that described for bile acid sequestrants and is insufficient to override the overall LDL lowering effects of ezetimibe.
General Description
Ezetimibe, (3R,4S)-1-(4-fluorophenyl)-3-((3S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)-2-azetidinone (Zetia), is an antihyperlipidemicagent that has usefulness in lowering cholesterol levels. Itacts by decreasing cholesterol absorption in the intestine byblocking the absorption of the sterol at the Brush boarder.Specifically, the -lactam binds to the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract that isresponsible for cholesterol absorption. Although it may beused alone, it is marketed as a combination product withsimvastatin under the trade name Vytorin.
Biochem/physiol Actions
Ezetimibe is a non statin drug that reduces intestinal cholesterol absorption. In addition, it also has an ability to reduce the risk of cardiovascular events in patients who had had an acute coronary syndrome and whose low-density lipoprotein (LDL) cholesterol values were within guideline recommendations.
Pharmacokinetics
Ezetimibe was shown to reduce the levels of total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apoprotein B (Apo B), non-high-density lipoprotein cholesterol (non-HDL-C), and triglycerides (TG), and increase high-density lipoprotein cholesterol (HDL-C) in patients with hyperlipidemia. This therapeutic effect was more profound when ezetimibe was co-administered with a statin or fenofibrate compared to either treatment alone.
In clinical trials involving patients with homozygous and heterozygous familial hypercholesterolemia and in those with sitosterolemia, a recommended therapeutic dose of ezetimibe was effective in reducing the LDL levels by 15-20% while increasing HDL-C by 2.5-5%.
Pharmacokinetics
Ezetimibe is administered orally; however, its absolute bioavailability cannot be determined because of its aqueous insolubility and the lack of an injectable formulation. Based on area under the curve values, the oral absorption ranges from 35 to 60%. Mean peak concentrations of the active glucuronidated metabolite are reached within 1 to 2 hours. Both ezetimibe and its glucuronide conjugate are extensively bound (>90%) to plasma proteins. The relative plasma concentrations of ezetimibe and its glucuronide conjugate range from 10 to 20% and from 80 to 90%, respectively. Both compounds have a long half-life of approximately 22 hours. The coadministration of food with ezetimibe has no effect on the extent of absorption.
Clinical Use
Ezetimibe is indicated as monotherapy or in combination with an HMGRI for the reduction of elevated total cholesterol, LDL cholesterol, and apoB in patients with primary (heterozygous familial and nonfamilial) hypercholesterolemia. When used as monotherapy, ezetimibe reduces LDL cholesterol by approximately 18%. When used in combination therapy with an HMGRI, LDL levels are reduced by 25 to 65% depending on the dose of the HMGRI inhibitor. Ezetimibe also is indicated for homozygous familial hypercholesterolemia in combination with either atorvastatin or simvastatin and for homozygous familial sitosterolemia. All indications are for patients who have not responded to diet, exercise, and other nonpharmacological methods.
Side Effects
Ezetimibe generally is well tolerated. The most common adverse effects are listed above. Whenever ezetimibe is used in combination with an HMGRI, the incidence of myopathy or rhabdomyolysis does not increase above that seen with HMGRI monotherapy.
Synthesis
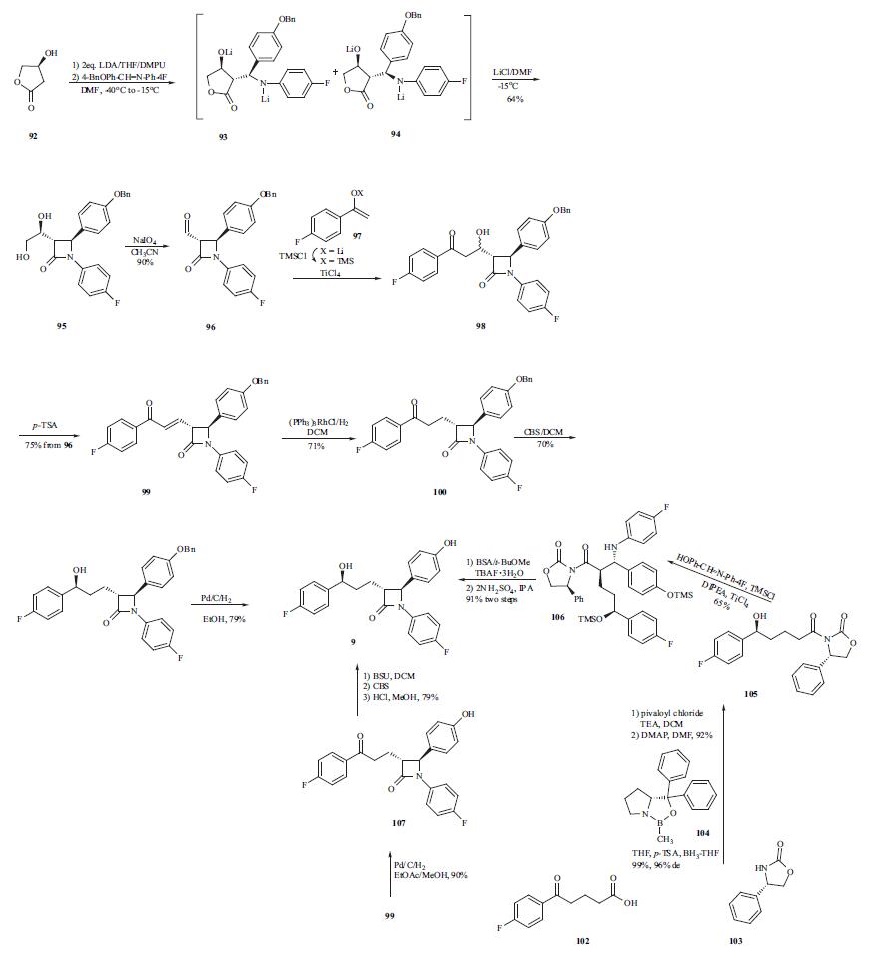
The synthesis of ezetimibe (9) begins with the one-step diastereoselective and practical synthesis of the trans |?- lactam from commercially available (S)-3-hydroxy-|?-lactone (92). Lactam 95 was obtained by generation of a dianion of lactone 92 with LDA in THF followed by addition of the imine and N,N?ˉ-dimethylpropyleneurea (DMPU) to give predominately adduct 93 (93:94 = 79:21). However, intermediate 93 and 94 did not cyclize to their respective lactams due to formation of stable lithium aggregates.Addition of lithium chloride/DMF was employed to cyclize the intermediates into trans-lactam 95 as the major product (trans:cis = 95:5) in a one-pot process from 92 in 64% yield. The 95:5 ratio of compound 95 was oxidatively cleaved with NaIO4 to give aldehyde 96. Mukaiyama aldol condensation was adopted to elaborate the 4-fluorophenylpropyl side chain to give alcohol 98. Without isolation, the reaction mixture was subjected to dehydration using p-TSA to give enone 99 in 75% yield from compound 96. Reduction of the double bond in 99 with Wilkinson?ˉs catalyst yielded ketone 100, which was subjected to the highly enantioselective CBS reduction to give alcohol 101 with a 98:2 selectivity of S:R at the benzylic position. Catalytic hydrogenation of compound 101 gave ezetimibe (9) in 79% yield. Alternatively, a palladium-catalyzed double reduction in EtOAc/MeOH of both the double bond and the benzyl protecting group in enone 99 produced free phenol 107 in 90% yield. A three-step one-pot procedure was subsequently developed to transform 107 into ezetimibe (9) in 79% yield. That is, free phenol 107 was protected in situ as its TMS ether using BSU followed by a highly selective CBS reduction of the ketone group to give the desired alcohol in 97% ee. The TMS group was removed during acidic workup to give ezetimibe (9). A more convergent approach to this drug was also developed by preparing the (S)-hydroxy side chain before the ring construction. Therefore, p-fluorobenzoylbutyric acid (102) was reacted with pivaloyl chloride and the acid chloride thus obtained was acylated with chiral auxiliary 103 to give the corresponding amide. The ketone group in the amide was reduced with (R)-MeCBS/BH3-THF (104) in the presence of p-TSA to give desired alcohol 105 in high yield (99%) and stereoselectivity (96 % d.e.). Chiral alcohol 105 was then mixed with the imine in the presence of TMSCl and DIPEA to protect the alcohols as TMS ethers. In the same pot, TiCl4 was added to catalyze the condensation reaction and gave compound 106 in 65% yield. Compound 106 was reacted with TBAF and a fluoridecatalyzed cyclization took place to give the corresponding lactam. Finally, the TMS protecting group was removed under acidic conditions to give ezetimibe (9) in 91% yield over two steps.
Drug interactions
Potentially hazardous interactions with other drugs Ciclosporin: concentration of both drugs possibly increased. Lipid lowering agents: avoid with fibrates; concentration of rosuvastatin increased - reduce rosuvastatin dose.
Metabolism
In humans, ezetimibe is rapidly and extensively metabolized via a phase II glucuronide conjugation reaction in the small intestine and liver to form its main phenolic metabolite, ezetimibe glucuronide. The main human liver and/or intestinal uridine 5′-diphosphate (UDP)-glucuronosyltransferase (UGT) enzymes responsible for the glucuronidation of ezetimibe were shown to be UGT1A1, 1A3, and 2B15 in vitro. Minimal phase I reaction involving oxidation of ezetimibe also occurs to form SCH 57871, and human jejunum microsomes also produced trace levels of a benzylic glucuronide (SCH 488128). Ezetimibe glucuronide accounts for 80-90% of the total circulating compound in plasma, and retains some pharmacological activity in inhibiting intestinal cholesterol uptake. In humans, ezetimibe and ezetimibe-glucuronide constitutes approximately 93% of the total drug in plasma. Plasma concentration-time profiles exhibit multiple peaks, suggestive of enterohepatic recycling, and about 20% of the drug distributed is reabsorbed due to enterohepatic recirculation.
Metabolism
Following oral administration, ezetimibe is rapidly and extensively metabolized in the intestinal wall and the liver to its active metabolite, a corresponding phenol glucuronide. This glucuronide is reexcreted in the bile back to its active site. A small amount (<5%) of ezetimibe undergoes oxidation to covert the benzylic hydroxyl group to a ketone; however, ezetimibe does not appear to exert any significant effect on the activity of CYP450 enzymes.
Side Effects
Ezetimibe may increase the risk of liver damage (when taken with a "statin") or muscle damage when taken with a fibrate or "statin". Tell your doctor right away if you experience any of the following symptoms: signs of liver problems (such as nausea/vomiting that doesn't stop, severe stomach/abdominal pain, yellowing eyes/skin, dark urine), muscle pain/tenderness/weakness (especially with fever or unusual tiredness).
References
1) Wang?et al. (2007),?Regulation of intestinal cholesterol absorption; Annu. Rev. Physiol.,?69?221 2) Garcia-Calvo?et al. (2005),?The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1); Proc. Natl. Acad. Sci. USA,?102?8132 3) Osuna-Ramos?et al.?(2018),?Ezetimibe inhibits dengue virus infection in Huh-7 cells by blocking the cholesterol transporter Niemann-Pick C1-like 1 receptor; Regulat. Curr. Opin. Cell Biol.,?160?151 4) Kim?et al.?(2017),?Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition; Autophagy,?13?1767
Properties of Ezetimibe
| Melting point: | 164-166°C |
| alpha | D22 -33.9° (c = 3 in methanol) |
| Boiling point: | 654.9±55.0 °C(Predicted) |
| Density | 1.334±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 15 mg/ml) |
| form | powder |
| pka | 9.72±0.30(Predicted) |
| color | White or off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 163222-33-1(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Azetidinone, 1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-, (3R,4S)- (163222-33-1) |
Safety information for Ezetimibe
Computed Descriptors for Ezetimibe
Ezetimibe manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](https://img.chemicalbook.in/CAS/GIF/189028-93-1.gif)
![Methyl (3R,4S)-1-(4-fluorophenyl)-2-oxo-4-[4-(phenylmethoxy)phenyl]-3-azetidinepropanoate](https://img.chemicalbook.in/CAS/GIF/204589-80-0.gif)
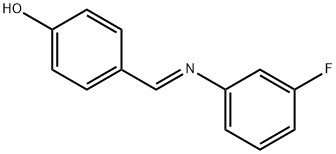
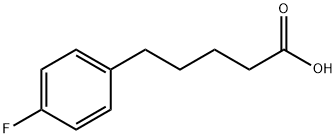
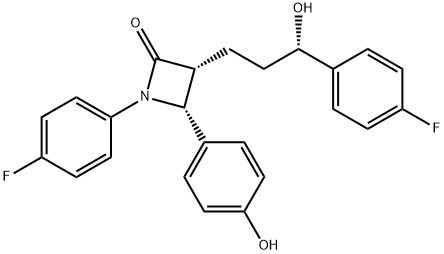
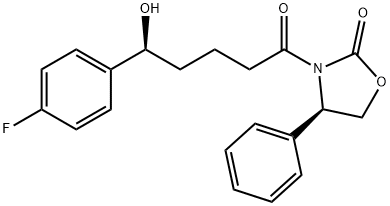
You may like
-
 Ezetimibe 98%View Details
Ezetimibe 98%View Details
163222-33-1 -
 163222-33-1 Ezetimibe 98%View Details
163222-33-1 Ezetimibe 98%View Details
163222-33-1 -
 Ezetimibe 98%View Details
Ezetimibe 98%View Details -
 163222-33-1 98%View Details
163222-33-1 98%View Details
163222-33-1 -
 Ezetimibe 98%View Details
Ezetimibe 98%View Details
163222-33-1 -
 Ezetimibe 99%View Details
Ezetimibe 99%View Details -
 EZETIMIBE 95-99 %View Details
EZETIMIBE 95-99 %View Details
163222-33-1 -
 Ezetimibe 95% CAS 163222-33-1View Details
Ezetimibe 95% CAS 163222-33-1View Details
163222-33-1
