PROADIFEN HYDROCHLORIDE
Synonym(s):α-Phenyl-α-propylbenzeneacetic acid 2-(diethylamino)ethyl ester;N,N-Diethylaminoethyl 2,2-diphenylvalerate;Proadifen;SKF-525A;SKF-525A, Hydrochloride - CAS 62-68-0 - Calbiochem
- CAS NO.:62-68-0
- Empirical Formula: C23H32ClNO2
- Molecular Weight: 389.96
- MDL number: MFCD00055151
- EINECS: 688-917-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
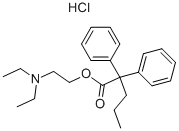
What is PROADIFEN HYDROCHLORIDE?
Description
SKF 525A is a widely used, nonspecific cytochrome P (CYP)450 inhibitor that demonstrates 100% inhibition of the various CYP450 isoforms at 1-
Chemical properties
Off-White Crystalline Solid
The Uses of PROADIFEN HYDROCHLORIDE
anti-infective
The Uses of PROADIFEN HYDROCHLORIDE
An inhibitor of NOS1, AChR, cytochrome P-450, and KIR6.1.
The Uses of PROADIFEN HYDROCHLORIDE
Proadifen hydrochloride is an inhibitor of NOS1, AChR, cytochrome P-450, and KIR6. Proadifen hydrochloride bind to the protein moiety of P-450, also reduced the hypoxic response. of Proadifen hydrochloride have good antiarrhythmic effects. SKF-525A substantially reduced the depletion of cardiac norepinephrine.
The Uses of PROADIFEN HYDROCHLORIDE
Cytochrome P-459 inhibitor; blocks glibenclamide-sensitive K+ channels; inhibits neuronal nitric oxide synthetase; stimulates endothelial cell prostacyclin while inhibiting platelet thromboxane synthesis
What are the applications of Application
Proadifen hydrochloride is an inhibitor of NOS1, AChR, cytochrome P-450, and KIR6.1
Safety Profile
Poison by intraperitoneal, subcutaneous, and intravenous routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and HCl.
in vitro
previous study found that when incubated with human liver microsomes, skf525a could undergo cyp-dependent oxidative n-deethylation to its secondary amine metabolite skf8742. in addition, skf525a and its metabolite and primary amine analog all inhibited cyp2b6-, cyp2c9-, cyp2c19-, cyp2d6-, as well as cyp3a-selective reactions to various degrees but had little effect on cyp1a2, cyp2a6, and cyp2e1 reactions [1].
in vivo
animal study found that skf 525a at 1.5-9 mg/kg could reduce or abolish the hypertensive effect of mcn-a-343, dmpp and nicotine, but could neither noticeably affect the hypertensive effect of tyramine, adrenaline and noradrenaline, nor the hypotensive effect of acetylcholine and orciprenaline. thus, skf 525a was able to block the rat sympathetic ganglia and the adrenal medulla and such blockade was non-specific. moreover, the blockade might result from the stabilizing effect of skf 525a on postsynaptic membranes of the sympathetic ganglia and the adrenal medulla [2].
References
1) Franklin and Hathaway (2008)?2-Diethylaminoethyl-2,2-diphenylvalerate-HCL (SKF525A) revisited: comparative cytochrome P450 inhibition in human liver microsomes by SKF525A, its metabolites, and SKF-acid and SKF-alcohol;?Drug Metab. Dispos,?36?2539 2) Suarez-Kurtz and Bianchi (1970)?Sites of action of SKF-525A in nerve and muscle;?J. Pharmacol. Exp. Ther.,?172?33 3) Spitzmaul?et al. (2009)?The local anesthetics proadifen and adiphenine inhibit nicotinic receptors by different molecular mechanisms; Br. J. Pharmacol.,?157?804 4) Prince and Sine (1999)?Acetylcholine and epibatidine binding to muscle acetylcholine receptors distinguish between concerted and uncoupled models; J. Biol. Chem.,?274?19623 5) Sakust and Yoneda (1994)?Inhibition by SKF 525A and quinacrine of endogenous glibenclamide-sensitive K+ channels in follicle-enclosed Xenopus oocytes; Eur. J. Pharmacol.,?252?117
Properties of PROADIFEN HYDROCHLORIDE
| Melting point: | 122-123°C |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 20 mg/ml) |
| form | White solid |
| color | Lustrous crystals from Me2CO/pet ether |
| Water Solubility | Soluble in methanol or water |
| Stability: | Store in Freezer |
| EPA Substance Registry System | Proadifen hydrochloride (62-68-0) |
Safety information for PROADIFEN HYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. |
Computed Descriptors for PROADIFEN HYDROCHLORIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
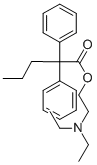
You may like
-
 Proadifen hydrochloride 97% CAS 62-68-0View Details
Proadifen hydrochloride 97% CAS 62-68-0View Details
62-68-0 -
 Proadifen hydrochloride CAS 62-68-0View Details
Proadifen hydrochloride CAS 62-68-0View Details
62-68-0 -
 SKF-525A, Hydrochloride CAS 62-68-0View Details
SKF-525A, Hydrochloride CAS 62-68-0View Details
62-68-0 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0