Virginiamycin
- CAS NO.:11006-76-1
- Empirical Formula: C71H84N10O17
- Molecular Weight: 1349.48346
- MDL number: MFCD01778146
- EINECS: 234-244-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
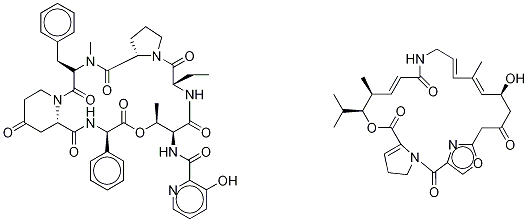
What is Virginiamycin?
Description
Mikamycin was found in the culture broth of Streptomyces mitakaensis by Umezawa et al. of the University of Tokyo in 1956. It is a mixture of two components, A and B, showing synergistic effects on each other. Staphylomycin, which was found by De Somer of Leuven University in 1955, is a closely related compound. Mikamycin shows strong activity against grampositive bacteria, including strains resistant to other antibiotics. It has been used topically to treat Staphylococcus or Streptococcusinfections of the skin, including burns.
The Uses of Virginiamycin
Pristinamycin is a mixture of two components that have a synergistic antibacterial action. The two components are Pristinamycin IIA (V673810) and Pristinamycin IA (P745000).
The Uses of Virginiamycin
Virginiamycin is a mixture of Virginiamycin S1 and Virginiamycin M1 (V673810). It is an antibiotic used in livestock feed to prevent disease among young swine. Also is used in ethanol production to prevent microbial contamination.
The Uses of Virginiamycin
Virginiamycin complex is defined as a mixture of 75% ostreogrycin A (virginiamycin M1) and 25% virginiamycin S1, together with the less abundant S analogues. As the two major components have quite different solubilities, these proportions are not readily achieved or used. BioAustralis has isolated and re-combined the individual components to provide the defined components of virginiamycin complex. The composition of the complex is important as Virginiamycin S1 acts a synergist, binding to the conformational change of the peptidyl transferase centre of the 50S ribosome induced by ostreogrycin A.
The Uses of Virginiamycin
Occupational dermatitis was observed in the pharmaceutical industry and in a surgeon who used topical virginiamycin on postoperative wounds (CJ Le Coz, unpublished observations).
What are the applications of Application
Virginiamycin Complex is a mixture of two streptogramin antibiotics, Virginiamycins M1 and S1, that act synergistically to inhibit protein synthesis in bacteria.
brand name
Stafac (SmithKline Beecham Animal Health).
Pharmaceutical Applications
A natural product of Streptomyces virginiae with antimicrobial activity similar to that of other streptogramins. It has chiefly been used as an animal feed additive, but is available in some countries for oral administration and in topical preparations.
Pharmaceutical Applications
The two major components are pristinamycin IA and pristinamycin IB. It is available in some countries for the oral treatment of upper respiratory, bronchopulmonary, dental, skin, genital and bone infections caused by susceptible organisms. In-vitro activity is summarized in Table 28.1. The drug is usually well tolerated, although minor gastrointestinal disturbances and rash may occur.
Biological Activity
virginiamycin complex is a a complex containing two streptogramin antibiotics.virginiamycin complex contains two streptogramin antibiotics, virginiamycin m1 (75%) and virginiamycin s1 (25%), produced by s. virginiae.
Contact allergens
Like the other streptogramin, pristinamycin, virginiamycin is made of two subunits, virginiamycin S1 and virginiamycin M1. Dermatitis was quite common in people using the formerly available topical virginiamycin. Occupational dermatitis was observed in the pharmaceutical industry, in breeders, and in a surgeon who used topical virginiamycin on postoperative wounds (personal observation).
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Used as an antibiotic.
in vitro
previous study found that the m1 and s1 components of virginiamycin (vm and vs) could inhibit protein synthesis in bacteria--reversibly when a single component was present and irreversibly when both were present. in cell-free systems, each factor bound to the large ribosomal subunit, and the affinity of ribosomes for vs was enhanced in the presence of vm. in addition, the binding of labeled vm to ribosomes yielded particles unable to perform poly(u)-directed polyphenylalanine synthesis. moreover, the association constant for the binding of vs to these particles was equal to that incubated with a mixture of vm and vs [1].
in vivo
a radiochemical method was developed to estimate cholyltaurine hydrolase potentials and rates of cholyltaurine hydrolysis in chicken intestinal homogenates. this method was used to monitor the effects of antibiotic feed additives on cholyltaurine hydrolase activity. results showed that virginiamycin could improve the rate of weight gain and feed conversion and decrease cholyltaurine hydrolase activity in ileal homogenates relative [2].
References
1. parfait, r., and cocito, c. lasting damage to bacterial ribosomes by reversibly bound virginiamycin m. proceedings of the national academy of sciences of the united states of america 77(9), 5492-5496 (1980).2. feighner, s.d., and dashkevicz, m.p. subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. applied and environmental microbiology 53(2), 331-336 (1987).
Properties of Virginiamycin
| Melting point: | 170-178℃ |
| storage temp. | Amber Vial, Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Pale Yellow |
| Stability: | Hygroscopic, Light Sensitive |
| EPA Substance Registry System | Virginiamycin (11006-76-1) |
Safety information for Virginiamycin
Computed Descriptors for Virginiamycin
Related products of tetrahydrofuran




You may like
-
 Pristinamycin CASView Details
Pristinamycin CASView Details -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3