Sodium ethoxide
Synonym(s):Sodium ethylate;Sodium ethylate, Sodium ethanolate
- CAS NO.:141-52-6
- Empirical Formula: C2H5NaO
- Molecular Weight: 68.05
- MDL number: MFCD00012417
- EINECS: 205-487-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
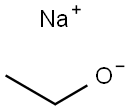
What is Sodium ethoxide?
Chemical properties
White powder, sometimes having brownish tinge; readily hydrolyzes to alcohol and sodium hydroxide.
Physical properties
White or yellowish powder; hygroscopic; darkens and decomposes on exposure to air; decomposes in water forming sodium hydroxide and ethanol; dissolves in absolute ethanol.
The Uses of Sodium ethoxide
Sodium ethoxide is used in organic synthesis for condensation reactions. It also is a catalyst in many organic reactions.
The Uses of Sodium ethoxide
Sodium ethoxide, 21% w/w in ethanol is used as a strong base in organic synthesis. It finds application in various chemical reactions such as condensation, esterification, alkoxylation and etherifcation. It is actively involved in Claisen condensation, Stobbe reaction and Wolf-kishner reduction. It is an important starting material for the synthesis of ethyl ester and diethyl ester of malonic acid. In Williamson ether synthesis, it reacts with ethyl bromide to form diethyl ether.
Definition
ChEBI: An organic monosodium salt that has ethoxide as the counterion.
Preparation
Sodium ethoxide is prepared by reacting sodium with absolute ethanol:
2Na + 2C2H5OH → 2C2H5ONa + H2
Sodium in small quantities is added to absolute alcohol at 10°C. The temperature is raised to warming (to about 38°C). The mixture is cooled again and sodium and absolute alcohol are added gradually followed by careful warming. The process is repeated to obtain a sufficient yield of the p
General Description
Sodium ethoxide is an alkoxide salt mainly used as a strong base in organic reactions such as deprotonation, dehydration and dehalogenation.
Hazard
As for caustic soda, ethanol. Sodium hydroxide is formed when sodium ethylate is exposed to moisture.
Flammability and Explosibility
Highly flammable
Purification Methods
It is a hygroscopic powder which should be stored under N2 in a cool place. A likely impurity is EtOH which can be removed by warming at 60-80o under high vacuum. It is hydrolysed by H2O to yield NaOH and EtOH. Other impurities, if kept in air for long periods are NaOH and Na2CO3. In this case the powder cannot be used if these impurities affect the reactivity, and a fresh sample should be acquired [IR: Seubold J Org Chem 21 156 1956]. [Beilstein 1 H 311, 1 IV 1289.]
Properties of Sodium ethoxide
| Melting point: | 260 °C |
| Boiling point: | 91°C |
| Density | 0.868 g/mL at 25 °C |
| vapor density | 1.6 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 48 °F |
| storage temp. | Store at +15°C to +25°C. |
| solubility | Soluble in ethanol and methanol. |
| form | Liquid |
| color | Yellow to brown |
| Specific Gravity | 0.868 |
| PH | 13 (5g/l, H2O, 20℃) |
| Water Solubility | Miscible |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 14,8539 |
| BRN | 3593646 |
| Exposure limits | ACGIH: STEL 1000 ppm OSHA: TWA 1000 ppm(1900 mg/m3) NIOSH: IDLH 3300 ppm; TWA 1000 ppm(1900 mg/m3) |
| Stability: | Reacts violently with acids, water. Incompatible with chlorinated solvents, moisture. Absorbs carbon dioxide from the air. Highly flammable. |
| CAS DataBase Reference | 141-52-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanol, sodium salt (141-52-6) |
Safety information for Sodium ethoxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H251:Self-heating substances and mixtures H314:Skin corrosion/irritation H371:Specific target organ toxicity, single exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium ethoxide
Sodium ethoxide manufacturer
Nasense Labs Private Limited
Supra Group of Companies
Syrohchem Laboratories Pvt Ltd
HYCHEM LABORATORIES
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Sodium ethoxide CAS 141-52-6View Details
Sodium ethoxide CAS 141-52-6View Details
141-52-6 -
 Sodium ethoxide CAS 141-52-6View Details
Sodium ethoxide CAS 141-52-6View Details
141-52-6 -
 Sodium ethoxide CAS 141-52-6View Details
Sodium ethoxide CAS 141-52-6View Details
141-52-6 -
 Sodium ethoxide, ~96% CAS 141-52-6View Details
Sodium ethoxide, ~96% CAS 141-52-6View Details
141-52-6 -
 Sodium Ethoxide CAS 141-52-6View Details
Sodium Ethoxide CAS 141-52-6View Details
141-52-6 -
 Sodium Ethoxide (ca. 20% in Ethanol) CAS 141-52-6View Details
Sodium Ethoxide (ca. 20% in Ethanol) CAS 141-52-6View Details
141-52-6 -
 Sodium ethoxide CAS 141-52-6View Details
Sodium ethoxide CAS 141-52-6View Details
141-52-6 -
 Sodium ethoxide CASView Details
Sodium ethoxide CASView Details