Primaquine diphosphate
Synonym(s):8-(4-Amino-1-methylbutylamino)-6-methoxyquinoline diphosphate salt;Primaquine bisphosphate;Primaquine diphosphate salt
- CAS NO.:63-45-6
- Empirical Formula: C15H27N3O9P2
- Molecular Weight: 455.34
- MDL number: MFCD00013166
- EINECS: 200-560-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
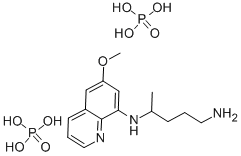
What is Primaquine diphosphate?
Chemical properties
orange powder
The Uses of Primaquine diphosphate
Primaquine diphosphate is an established antimalarial drug used in the treatment of persistent liver forms of P. vivax or P. ovale infection for its ability to kill late-stage gametocytes and hypnozoites. In historical terms, this aminoquinoline was the stimulus for the discovery of glucose-6-phosphate (G6P) dehydrogenase deficiency as it induced hemolytic anemia in patients lacking the G6P-metabolizing enzyme.
The Uses of Primaquine diphosphate
Antimalarial.
The Uses of Primaquine diphosphate
acetylcholinesterase inhibitor
What are the applications of Application
Primaquine phosphate is an antimalarian agent that is also an inhibitor of cardiac Na+ current
brand name
Primaquine (Sanofi Aventis).
Biological Activity
primaquine, an 8-aminoquinoline, is introduced as a curative antimalarial agent in 1950. since then, the drug has been applied extensively to against the exoerythrocytic stage of malaria. it is demonstrated tthat primaquine, by binding to nucleic acids, could therefore block protein synthesis, alter lipid synthesis and interact with biological membranes. [1]
Clinical Use
Treatment of malaria (Plasmodium vivax and
Plasmodium ovale), in combination with chloroquine
Treatment of Pneumocystis jiroveci pneumonia
(PCP), in combination with clindamycin
in vitro
chicken embryo cells (cec) model were adopted to investigate the effect of primaquine on newcastle disease virus replication. it was found that virus-induced hemadsorption was inhibited by primaquine in a dose-dependent manner and was completely suppressed by primaquine 250 g/ml. viral ribonucleic acid (rna) synthesis was found to be suppressed when primaquine was added early in the virus replication cycle. whereas, when the drug was added late in the cycle, rna synthesis was stimulation. [1]
in vivo
primaquine liposomes were labelled by 99mtc and injected intravenously to swiss albino mice. after injection, the major accumulation organ of liposomes was liver followed by spleen, pancreas, lungs and the others. findings also suggested that primaquine could block the eradication of the parasites and prevent relapse by destruction of the exoerythrocytic liver stages. [2]
Drug interactions
Potentially hazardous interactions with other drugs
Antimalarials: avoid concomitant use with
artemether/lumefantrine.
Metabolism
Rapidly metabolised in the liver. Its major metabolite carboxyprimaquine accumulates in the plasma on repeated dosage but possesses less antimalarial activity than the parent compound. Little unchanged drug is excreted in the urine.
Purification Methods
It forms yellow crystals from 90% aqueous EtOH and is moderately soluble in H2O. The oxalate salt has m 182.5-185o (from 80% aqueous EtOH), and the free base is a viscous liquid b 165-170o/0.002mm, 175-177o/2mm. [Elderfield et al. J Am Chem Soc 68 1526 1964, Elderfield et al. J Am Chem Soc 77 4817 1955, Beilstein 22 III/IV 5817.]
References
[1]burdick jr and durand dp. primaquine diphosphate: inhibition of newcastle disease virus replication. antimicrob agents ch. 1974 oct 15; 6(4): 460-4.
[2]aricat b, ozert ay, ercans mt and hincalt aa. characterization, in vitro and in vivo studies on primaquine diphosphate liposomes. j. microencapsulation. 1995; 12(5): 469-85.
[3]soto j, toledo j, rodriquez m, sanchez j, herrera r, padilla j and berman j. double-blind, randomized, placebo-controlled assessment of chloroquine/primaquine prophylaxis for malaria in nonimmune colombian soldiers. clin infect dis. 1999 jul; 29: 199-201.
Properties of Primaquine diphosphate
| Melting point: | 205-206 °C (dec.)(lit.) |
| storage temp. | 2-8°C |
| solubility | water: soluble50mg/mL, clear, orange to red |
| form | neat |
| form | Solid |
| color | Red brown to orange |
| Water Solubility | moderately soluble |
| Merck | 13,7833 |
| BRN | 3812133 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 63-45-6(CAS DataBase Reference) |
Safety information for Primaquine diphosphate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Primaquine diphosphate
Primaquine diphosphate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 63-45-6 98%View Details
63-45-6 98%View Details
63-45-6 -
 Primaquine phosphate 63-45-6 99%View Details
Primaquine phosphate 63-45-6 99%View Details
63-45-6 -
 63-45-6 Primaquine phosphate 98%View Details
63-45-6 Primaquine phosphate 98%View Details
63-45-6 -
 Primaquine phosphate 97% CAS 63-45-6View Details
Primaquine phosphate 97% CAS 63-45-6View Details
63-45-6 -
 Primaquine Phosphate CAS 63-45-6View Details
Primaquine Phosphate CAS 63-45-6View Details
63-45-6 -
 Primaquine phosphate CAS 63-45-6View Details
Primaquine phosphate CAS 63-45-6View Details
63-45-6 -
 Primaquine bisphosphate CAS 63-45-6View Details
Primaquine bisphosphate CAS 63-45-6View Details
63-45-6 -
 Primaquine diphosphate CAS 63-45-6View Details
Primaquine diphosphate CAS 63-45-6View Details
63-45-6
