Calcium phosphate dibasic dihydrate
Synonym(s):Calcium hydrogen phosphate dihydrate;Calcium phosphate dibasic dihydrate
- CAS NO.:7789-77-7
- Empirical Formula: CaH7O5P
- Molecular Weight: 158.1
- MDL number: MFCD00149621
- EINECS: 616-542-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41
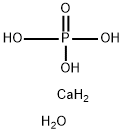
What is Calcium phosphate dibasic dihydrate?
Chemical properties
white crystalline solid
Chemical properties
Dibasic calcium phosphate dihydrate is a white, odorless, tasteless powder or crystalline solid. It occurs as monoclinic crystals.
The Uses of Calcium phosphate dibasic dihydrate
Dicalcium phosphate dihydrate is only slightly soluble at ordinary temperatures of mixing and holding doughs and batters. As a result, it does not release acidity for reaction with soda until late in the baking stage, when the temperature reaches 135 to 140°F. Since DCP·2H20 does not begin to react below 135°F, and the interior structure of a baked product begins to firm at about 160°F, a product that bakes rapidly may not provide sufficient time for complete release of all the C02. DCP·2H2 0, therefore, cannot be used in biscuits, pancakes or any baked product that is completely baked in less than 20 min.
Dicalcium phosphate dihydrate is seldom used by itself in leavening systems but is usually combined with fasterreacting acidic phosphates. Its major applications are in cake mixes, frozen bread doughs, and other products requiring a half hour or more to complete baking. It has a low neutralizing value, and therefore more DCP·2H20 is required to neutralize a given amount of soda than for other phosphate-leavening acids.
The Uses of Calcium phosphate dibasic dihydrate
Replenisher (calcium); pharmaceutic aid (tablet base).
The Uses of Calcium phosphate dibasic dihydrate
Dicalcium Phosphate, Dihydrate is a source of calcium and phosphorus that also functions as a dough conditioner and bleaching agent. It functions as a dough conditioner in bakery products, as a bleaching agent in flour, as a source of calcium and phosphorus in cereal products, and as a source of calcium for alginate gels. It contains approximately 23% calcium. It is practically insoluble in water. It is also termed dibasic calcium phosphate, dihydrate and calcium phosphate dibasic, hydrous. It is used in dessert gels, baked goods, cereals, and breakfast cereals.
Indications
For use as an over the counter calcium and phosphate supplement, antacid, or a source of calcium and phosphate in toothpaste .
Production Methods
Calcium phosphates are usually manufactured by reacting very pure phosphoric acid with calcium hydroxide, Ca(OH)2 obtained from limestone, in stoichiometric ratio in aqueous suspension followed by drying at a temperature that will allow the correct hydration state to be achieved. After drying, the coarse-grade material is obtained by means of a classification unit; the fine particle-size material is obtained by milling.
Definition
ChEBI: Calcium hydrogenphosphate dihydrate is a calcium salt and a hydrate.
brand name
CalStar (FMC); D.C.P. (Parke-Davis).
Pharmaceutical Applications
Dibasic calcium phosphate dihydrate is widely used in tablet
formulations both as an excipient and as a source of calcium and
phosphorus in nutritional supplements. It is one of the more
widely used materials, particularly in the nutritional/health food
sectors. It is also used in pharmaceutical products because of its
compaction properties, and the good flow properties of the coarsegrade
material. The predominant deformation mechanism of
dibasic calcium phosphate coarse-grade is brittle fracture and this
reduces the strain-rate sensitivity of the material, thus allowing
easier transition from the laboratory to production scale. However,
dibasic calcium phosphate dihydrate is abrasive and a lubricant is
required for tableting, for example about 1% w/w of magnesium
stearate or about 1% w/w of sodium stearyl fumarate is commonly
used.
Two main particle-size grades of dibasic calcium phosphate
dihydrate are used in the pharmaceutical industry. The milled
material is typically used in wet-granulated, roller-compacted or
slugged formulations. The ‘unmilled’ or coarse-grade material is
typically used in direct-compression formulations.
Dibasic calcium phosphate dihydrate is nonhygroscopic and
stable at room temperature. However, under certain conditions of
temperature and humidity, it can lose water of crystallization below
1008℃. This has implications for certain types of packaging and
aqueous film coating since the loss of water of crystallization
appears to be initiated by high humidity and by implication high
moisture vapor concentrations in the vicinity of the dibasic calcium
phosphate dihydrate particles.
Dibasic calcium phosphate dihydrate is also used in toothpaste
and dentifrice formulations for its abrasive properties.
Pharmacokinetics
Calcium phosphate reacts with acid in the stomach to raise the pH . In toothpaste it provides a source of calcium and phosphate ions to support remineralization of the teeth . As a supplement it provides a source of calcium and phospate, both of which are important ions in bone homeostasis.
Safety
Dibasic calcium phosphate dihydrate is widely used in oral pharmaceutical products, food products, and toothpastes, and is generally regarded as a nontoxic and nonirritant material. However, oral ingestion of large quantities may cause abdominal discomfort.
Metabolism
Not Available
Storage
Dibasic calcium phosphate dihydrate is a nonhygroscopic, relatively
stable material. However, under certain conditions the dihydrate
can lose water of crystallization. This has implications for both
storage of the bulk material and coating and packaging of tablets
containing dibasic calcium phosphate dihydrate.
The bulk material should be stored in a well-closed container in a
cool, dry place.
Purification Methods
Crystallise it from a near-saturated solution in 50% aqueous reagent grade phosphoric acid at 100o by filtering through fritted glass and cooling to room temperature. The crystals are filtered off, and this process is repeated three times using fresh acid. For the final crystallisation the solution is cooled slowly with constant stirring to give thin plate crystals that are filtered off on a fritted glass funnel, washed free of acid with anhydrous acetone and dry in a vacuum desiccator [Egan et al.J Am Chem Soc 78 1811 1956].
Incompatibilities
Dibasic calcium phosphate dihydrate should not be used to formulate tetracycline antibiotics. Dibasic calcium phosphate dihydrate has been reported to be incompatible with indomethacin, aspirin, aspartame, ampicillin, cephalexin, and erythromycin. The surface of dibasic calcium phosphate dihydrate is alkaline and consequently it should not be used with drugs that are sensitive to alkaline pH.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in nonparenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Calcium phosphate dibasic dihydrate
| Melting point: | 109°C -H₂O |
| Density | 2.31 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Practically insoluble in water and in ethanol (96 per cent). It dissolves in dilute hydrochloric acid and in dilute nitric acid. |
| form | Solid |
| color | White to almost white |
| Water Solubility | Slightly soluble in water. Soluble in dilute hydrochloric, nitric, and acetic acid. Insoluble in alcohol |
| Stability: | Stable. Incompatible with acids. |
| CAS DataBase Reference | 7789-77-7(CAS DataBase Reference) |
Safety information for Calcium phosphate dibasic dihydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Calcium phosphate dibasic dihydrate
| InChIKey | XAAHAAMILDNBPS-UHFFFAOYSA-L |
Calcium phosphate dibasic dihydrate manufacturer
Jaiswal S Cyber Shop
Imex Overseas
Halogens
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Dicalcium Phosphate - Dihydrate 98%View Details
Dicalcium Phosphate - Dihydrate 98%View Details -
 Calcium hydrogenphosphate dihydrate 98%View Details
Calcium hydrogenphosphate dihydrate 98%View Details -
 Calcium hydrogen phosphate dihydrate CAS 7789-77-7View Details
Calcium hydrogen phosphate dihydrate CAS 7789-77-7View Details
7789-77-7 -
 Calcium hydrogen phosphate dihydrate CAS 7789-77-7View Details
Calcium hydrogen phosphate dihydrate CAS 7789-77-7View Details
7789-77-7 -
 Calcium hydrogen phosphate, dihydrate, 98%+ CAS 7789-77-7View Details
Calcium hydrogen phosphate, dihydrate, 98%+ CAS 7789-77-7View Details
7789-77-7 -
 Calcium phosphate dibasic dihydrate CAS 7789-77-7View Details
Calcium phosphate dibasic dihydrate CAS 7789-77-7View Details
7789-77-7 -
 CALCIUM HYDROGEN PHOSPHATE DIHYDRATE Extra Pure CAS 7789-77-7View Details
CALCIUM HYDROGEN PHOSPHATE DIHYDRATE Extra Pure CAS 7789-77-7View Details
7789-77-7 -
 JCSSUPER 7789-77-7 di-Calcium phosphate dihydrate extrapure 500 gm., Powdered, Packaging Type: BottleView Details
JCSSUPER 7789-77-7 di-Calcium phosphate dihydrate extrapure 500 gm., Powdered, Packaging Type: BottleView Details
7789-77-7
