Potassium bromate
Synonym(s):Bromic Acid, Potassium Salt;Potassium bromate
- CAS NO.:7758-01-2
- Empirical Formula: BrKO3
- Molecular Weight: 167
- MDL number: MFCD00011359
- EINECS: 231-829-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:51
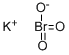
What is Potassium bromate ?
Description
Potassium bromate (KBrO3) is a typical potassium salt in that it is water-soluble and has a high melting point. The bromate anion is a potent oxidizer.
KBrO3‘s only significant commercial use is as a “flour improver” in bakeries. According to the Professional Baker’s Reference in the King Arthur Flour Web site, it “strengthens dough and allows for greater oven spring and higher rising in the oven.” The reference states that KBrO3 is a “slow-acting” oxidizer, which means that it is effective during the mixing, fermentation, and proofing stages of the baking process.
Used properly, KBrO3 is completely consumed by the end of baking. But because good baking practices may not always be used, its carcinogenicity (see hazard information box), prompted its ban in the early 2000s in the European Union, Canada, China, South Korea, and some South American countries. Its use is still legal in the United States, but California’s Proposition 65 law dictates that bromated flour must be labeled as a carcinogen.
Chemical properties
Potassium bromate is a white crystalline solid.
The Uses of Potassium bromate
Potassium Bromate is a dough conditioner that exists as white crystals or powder and is soluble in water. it exists in the anhydrous form as white granular powder and in the hydrated form as small white crystals or granules. it is used to age and improve the baking properties of flour. it is used with potassium iodate and azodicarbon- amide to modify the protein in bread flour to promote the desired properties of loaf volume and shape. it is used in baked goods.
The Uses of Potassium bromate
It is used in oxidizing agent in acid solutions.
The Uses of Potassium bromate
Bread- and flour-improving agent; in analytical chemistry.
What are the applications of Application
Potassium bromate is a powerful brominating agent for deactivated aromatics
Definition
ChEBI: Potassium bromate is a potassium salt and a bromate salt. It has a role as a flour treatment agent.
General Description
A white crystalline solid.
Air & Water Reactions
Water soluble.
Reactivity Profile
Potassium bromate is a strong oxidizing agent. Forms very flammable mixtures with combustible materials. Such mixtures may be explosive if the combustible material is finely divided and are often ignitable by friction. A mixture with finely divided aluminum can explode by heat, percussion, and friction [Mellor 2:310. 1946-47]. Reacts explosively with selenium [Mellor 2 Supp1:763. 1956]. Prolonged exposure to fire or heat can result in an explosion .
Hazard
Dangerous fire risk in contact with organic materials, strong irritant. Possible carcinogen.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. A poison by ingestion. A powerful oxidzer. An irritant to skin, eyes, and mucous membranes. Mutation data reported. Wxtures with sulfur may ignite. Violent reaction with Al, Al + dmitrotoluene @ 290°, As, C, Cu, Pb(C2H3O2)2, metal sulfides, organic matter, P, S. Aqueous solutions react violently with selenium. When heated to decomposition it emits very toxic fumes of Brand K2O. See also BROMIDES.
Potential Exposure
Potassium bromate is used as animal feed additive, food additive; flavor and packaging material; as a laboratory reagent; an oxidizing agent.
Shipping
UN1479 Potassium bromate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Crystallise KBrO3 from distilled H2O (2mL/g) between 100o and 0o. To remove bromide contamination, a 5% solution in distilled H2O, cooled to 10o, is bubbled with gaseous chlorine for 2hours, then filtered and extracted with reagent grade CCl4 until colourless and odourless. After evaporating the aqueous phase to about half its volume, it is cooled again slowly to about 10o. The crystalline KBrO3 that separates, is washed with 95% EtOH and dried in a vacuum [Boyd et al. J Am Chem Soc 74 237 1952]. Another way to remove Br-ions is by stirring several times in MeOH and then drying at 150o [Field & Boyd J Phys Chem 89 3767 1985].
Incompatibilities
A strong oxidizer.Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/ friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide. Incompatible with aluminum, copper.
Properties of Potassium bromate
| Melting point: | 350 °C |
| Density | 3.27 |
| storage temp. | Store below +30°C. |
| solubility | Potassium bromate is highly soluble in water (7.5 g/100 mL at 25°C; 49.8 g/100 mL at 100°C), slightly soluble in ethanol, and almost insoluble in acetone; it is very stable when dissolved in water at room temperature. |
| form | Powder/Solid |
| appearance | white crystals or powder |
| color | White crystals or granules |
| Specific Gravity | 3.27 |
| PH | 5.0-9.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | 70 g/L (20 ºC) |
| Merck | 14,7617 |
| Stability: | Strong oxidizer - contact with combustible materials may cause fire. Incompatible with combustible material, organics, reducing agents, aluminium, finely powdered metals. |
| CAS DataBase Reference | 7758-01-2(CAS DataBase Reference) |
| IARC | 2B (Vol. Sup 7, 73) 1999 |
| EPA Substance Registry System | Potassium bromate (7758-01-2) |
Safety information for Potassium bromate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Potassium bromate
Potassium bromate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Potassium bromate 99%View Details
Potassium bromate 99%View Details -
 Potassium bromate 98%View Details
Potassium bromate 98%View Details -
 Potassium bromate CAS 7758-01-2View Details
Potassium bromate CAS 7758-01-2View Details
7758-01-2 -
 Potassium bromate/Potassium bromide CASView Details
Potassium bromate/Potassium bromide CASView Details -
 Potassium bromate/Potassium bromide CASView Details
Potassium bromate/Potassium bromide CASView Details -
 POTASSIUM BROMATE 99%View Details
POTASSIUM BROMATE 99%View Details -
 POTASSIUM BROMATE Extra Pure CASView Details
POTASSIUM BROMATE Extra Pure CASView Details -
 POTASSIUM BROMATE 0.01667M (0.1N) STANDARDIZED SOLUTION traceable to NIST CASView Details
POTASSIUM BROMATE 0.01667M (0.1N) STANDARDIZED SOLUTION traceable to NIST CASView Details
