Sodium dichromate dihydrate
Synonym(s):Sodium bichromate;Sodium bichromate, Sodium pyrochromate;Sodium dichromate dihydrate
- CAS NO.:7789-12-0
- Empirical Formula: Cr2H5NaO8
- Molecular Weight: 260.01
- MDL number: MFCD00149166
- EINECS: 616-541-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
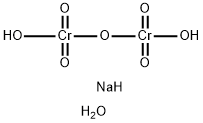
What is Sodium dichromate dihydrate?
Chemical properties
Orange-yellow powder. Soluble in acids and hot water; insoluble in alcohol and ether.
Chemical properties
Sodium chromate, including the hexahydrate, is yellow crystalline solids that can also be used in solution. Disodium dichromate (10588-01-9):
The Uses of Sodium dichromate dihydrate
Sodium Dichromate Dihydrate is used as a catalyst in the synthesis of Endoperoxide II. Strong oxidizing agent in organic synthesis. Used in metal finishing as an aid in corrosion resistance.
The Uses of Sodium dichromate dihydrate
It is used in preparation of titrant in redox titrations.
What are the applications of Application
Sodium dichromate dihydrate is a hydrate preparation of dichromate
Definition
A red crystallinesolid, Na2Cr2O7.2H2O, solublein water and insoluble in ethanol. Itis usually known as the dihydrate(r.d. 2.52), which starts to lose waterabove 100°C; the compound decomposesabove 400°C. It is made bymelting chrome iron ore with limeand soda ash and acidification of thechromate thus formed. Sodiumdichromate is cheaper than the correspondingpotassium compound buthas the disadvantage of being hygroscopic.It is used as a mordant in dyeing,as an oxidizing agent in organicchemistry, and in analytical chemistry.
General Description
Solid orange-yellow crystal or powder.
Air & Water Reactions
Water soluble (hot water).
Reactivity Profile
Sodium dichromate dihydrate can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Reactions may be rapid but often requires initiation (heat, spark, catalyst, addition of a solvent). Can react violently with active metals, cyanides, esters, and thiocyanates.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
INHALATION: Corrosive to mucous membranes continuous exposure may lead to perforation of nasal septum. EYES: Conjunctivitis and lacrimation. SKIN: Corrosive producing deep penetrating ulcers to exposed area. Slow to heal. INGESTION: Has a harsh metallic taste. May cause vertigo, thirst, abdominal pain, vomiting, shock, convulsions and coma.
Potential Exposure
Used to make dyes, inks, pigments, and other chromates; in leather tanning, a corrosion inhibitor in circulating water systems; metal treatment; a drilling mud additive; chemical intermediate for chromium catalysts; colorimetry, oxidizing agent; bleaching agent; an algicide, fungicide, insecticide; in wood preservation.
Shipping
UN3087 Oxidizing solid, toxic, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, Technical Name Required. UN3085 Oxidizing solid, corrosive, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, 8-Corrosive material, Technical Name Required.
Purification Methods
Crystallise the dichromate from small volumes of H2O by evaporation to crystallisation. Its solubility in H2O is 238% at 0o and 508% at boiling. The red dihydrate is slowly dehydrated by heating at 100o for long periods. It is deliquescent and is a powerful oxidising agent—do not place it in contact with skin— wash immediately as it is caustic. (Possible carcinogen.) Sodium dihydrogen orthophosphate ( 2 H2O) [13472-35-0 (2H2O), 10049-21-5 (H2O), 7558-80-7 (anhydrous)] M 156.0, m 60o(dec), d 4 1.91. Crystallise it from warm water (0.5mL/g) by chilling.
Incompatibilities
Aqueous solution in a base. A strong oxidizer. Violent reaction with reducing agents; combustibles, strong acids; organic materials.
Properties of Sodium dichromate dihydrate
| Melting point: | 91 °C(lit.) |
| Boiling point: | 400°C |
| Density | 2,348 g/cm3 |
| vapor density | 10 (vs air) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Methanol (Slightly, Sonicated) |
| form | Solid |
| color | Red-orange |
| Specific Gravity | 2.348 |
| PH Range | 3.5-4.0 |
| Odor | Odorless |
| Water Solubility | 730 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8609 |
| Dielectric constant | 2.9(Ambient) |
| CAS DataBase Reference | 7789-12-0(CAS DataBase Reference) |
| EPA Substance Registry System | Disodium dichromate dihydrate (7789-12-0) |
Safety information for Sodium dichromate dihydrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H312:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium dichromate dihydrate
Sodium dichromate dihydrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Sodium dichromate dihydrate, For analysis ACS CAS 7789-12-0View Details
Sodium dichromate dihydrate, For analysis ACS CAS 7789-12-0View Details
7789-12-0 -
 Sodium dichromate dihydrate, For analysis ACS CAS 7789-12-0View Details
Sodium dichromate dihydrate, For analysis ACS CAS 7789-12-0View Details
7789-12-0 -
 Sodium dichromate dihydrate CAS 7789-12-0View Details
Sodium dichromate dihydrate CAS 7789-12-0View Details
7789-12-0 -
 Sodium dichromate dihydrate CAS 7789-12-0View Details
Sodium dichromate dihydrate CAS 7789-12-0View Details
7789-12-0 -
 Sodium dichromate dihydrate CAS 7789-12-0View Details
Sodium dichromate dihydrate CAS 7789-12-0View Details
7789-12-0 -
 Sodium Dichromate Dihydrate pure CAS 7789-12-0View Details
Sodium Dichromate Dihydrate pure CAS 7789-12-0View Details
7789-12-0 -
 Sodium Dichromate Dihydrate extrapure AR CAS 7789-12-0View Details
Sodium Dichromate Dihydrate extrapure AR CAS 7789-12-0View Details
7789-12-0 -
 Sodium Dichromate CASView Details
Sodium Dichromate CASView Details
