Potassium binoxalate
- CAS NO.:127-95-7
- Empirical Formula: C2HKO4
- Molecular Weight: 128.13
- MDL number: MFCD00036407
- EINECS: 204-873-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-05 20:24:49
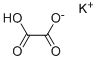
What is Potassium binoxalate?
Description
Potassium hydrogen oxalate, also known as potassium bioxalate, is a salt with formula KHC2O4 or K+·HO2C-CO2-. It is one of the most common salts of the hydrogenoxalate anion, and can be obtained by reacting potassium hydroxide with oxalic acid in 1:1 mole ratio.
The salt is also known as potassium hydrogen oxalate , acid potassium oxalate, or monobasic potassium oxalate. In older literature, it was also called sorrel salt, sal acetosella ,
Potassium hydrogen oxalate occurs in some plants, notably sorrel. It is a commercial product, used in photography, marble grinding, and to remove ink stains.
Chemical properties
The anhydrous product is a white, odorless, crystalline solid, hygroscopic and soluble in water (2.5 g/100 g at room temperature). The solutions are basic. Below 50 °C the much less soluble potassium tetraoxalate forms and precipitates out of solution.
The monohydrate KHC2O4·H2O starts losing the water at 100 °C.
The anhydrous salt was found to have remarkable elastic anisotropy, due to its crystal structure that consists of relatively rigid columns of hydrogen-bonded hydrogenoxalate anions, joined into sheets by ionic K–O bonds. .
History
Potassium acid oxalate, KHC2O4, exists as a monohydrate. It is of historical interest because it is the salt of sorrel found in vegetation and the first oxalate isolated.
The Uses of Potassium binoxalate
Removing ink stains, scouring metals, cleaning wood, photography, laboratory reagent, mordant.
General Description
Odorless white solid. Sinks in water.
Air & Water Reactions
Hygroscopic. Gives basic solution, below 50°C dissolves in water and reacts to form the much less soluble potassium tetraoxalate, which separates out.
Reactivity Profile
Salts, basic, such as Potassium binoxalate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Toxic by ingestion.
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion causes burning pain in throat, esophagus, and stomach; exposed areas of mucous membrane turn white; vomiting, severe purging, weak pulse, and cardiovascular collapse; if death is delayed, neuromuscular symptoms develop. Contact with dust irritates eyes and may cause mild irritation of skin.
Flammability and Explosibility
Non flammable
Toxicology
Potassium hydrogen oxalate is strongly irritating to eyes, mucoses and gastrointestinal tract. It may cause cardiac failure and death .
Safety Profile
Moderately toxic to humans by ingestion. Human systemic effects by ingestion and intravenous routes: general anesthetic, somnolence, fluid intake, blood pressure increase or decrease, esophagus changes,nausea or vomiting, and urine volume decrease or anu
Purification Methods
Crystallise it from H2O by dissolving 20g in 100mL H2O at 60o containing 4g of potassium oxalate, filtering and allowing to cool to 25o. The crystals, after washing three or four times with water, are allowed to dry in air. [Beilstein 2 III 1552.]
Properties of Potassium binoxalate
| Melting point: | >300°C |
| Density | 2.088 [HAW93] |
| vapor pressure | 0.002Pa at 20℃ |
| storage temp. | Room Temperature |
| solubility | DMSO |
| form | Solid |
| pka | 4.27[at 20 ℃] |
| color | Monoclinic, colorless crystals |
| Water Solubility | soluble 40 parts cold H2O, 6 parts boiling H2O [MER06] |
| Stability: | Stable. |
| CAS DataBase Reference | 127-95-7(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanedioic acid, potassium salt (1:1) (127-95-7) |
Safety information for Potassium binoxalate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Potassium binoxalate
Potassium binoxalate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 A.B.ENTERPRISES POTASSIUM BINOXALATE : (127-95-7 )View Details
A.B.ENTERPRISES POTASSIUM BINOXALATE : (127-95-7 )View Details
127-95-7 -
 Potassium Binoxalate (PBO)View Details
Potassium Binoxalate (PBO)View Details
127-95-7 -
 Potassium Binoxalate PowderView Details
Potassium Binoxalate PowderView Details
614-60-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
