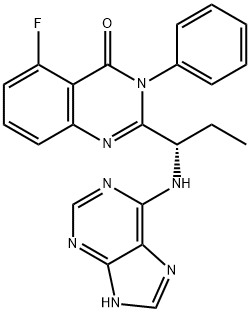
PLX3397 (Pexidartinib)
- CAS NO.:1029044-16-3
- Empirical Formula: C20H15ClF3N5
- Molecular Weight: 417.81
- MDL number: MFCD28900745
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is PLX3397 (Pexidartinib)?
Absorption
Following administration of single doses in healthy subjects and multiple doses in patients, the mean Cmax was 8625 ng/mL and the mean AUC was 77465 ngxh/mL. The median Tmax was 2.5 hours and the time to reach the steady state was approximately 7 days. Administration of pexidartinib with a high fat meal resulted in an increased drug Cmax and AUC by 100%, with a delay in Tmax by 2.5 hours.
Toxicity
There is limited human data on the overdose of pexidartinib. In 4-week toxicology studies, the no-observed-adverse-effect levels (NOAELs) of pexidatrtinib were determined to be 10 mg/kg/day in rats and 6 mg/kg/day in dogs. Pexidartinib was shown to cause hepatotoxicity in clinical trials, including mixed or cholestatic hepatotoxicity, and embryo-fetal toxicity in animal studies.
Description
Colony stimulating factor 1 (CSF1) is a cytokine that is involved in the recruitment and activation of tissue macrophages. It exerts these effects by binding to its corresponding receptor tyrosine kinase, the cFMS/CSF1 receptor (CSF1R). Pexidartinib is a brain-penetrant inhibitor of CSF1R, as well as c-Kit and FLT3 (IC50s = 20, 10, and 160 nM in vitro, respectively). It has been used in combination with paclitaxel to block macrophage recruitment in mammary tumor-bearing mice, thus slowing primary tumor development and metastasis. Pexidartinib has also been used to block microglial stimulation of glioblastoma invasion in both cell culture and a mouse model of glioblastoma multiforme.
The Uses of PLX3397 (Pexidartinib)
Pexidartinib is RTK inhibitor. It can be used in pharmacological activity, therapeutic use, and biological study of inhibition of colony stimulating factor-1 receptor improves antitumor efficacy of BRAF inhibition.
Background
Pexidartinib is a selective tyrosine kinase inhibitor that works by inhibiting the colony-stimulating factor (CSF1)/CSF1 receptor pathway. Pexidartinib was originally developed by Daiichi Sankyo, Inc. and it was approved by the FDA in August 2019 as the first systemic therapy for adult patients with symptomatic tenosynovial giant cell tumor. Tenosynovial giant cell tumor is a rare form of non-malignant tumor that causes the synovium and tendon sheaths to thicken and overgrow, leading to damage in surrounding joint tissue. Debilitating symptoms often follow with tenosynovial giant cell tumors, along with a risk of significant functional limitations and a reduced quality of life in patients.
While surgical resection is a current standard of care for tenosynovial giant cell tumor, there are tumor types where surgeries are deemed clinically ineffective with a high risk of lifetime recurrence. Pexidartinib works by blocking the immune responses that are activated in tenosynovial giant cell tumors. In clinical trials, pexidartinib was shown to promote improvements in patient symptoms and functional outcomes in TGCT. Pexidartinib is available in oral formulations and it is commonly marketed as Turalio.
Indications
Pexidartinib is indicated for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not amenable to improvement with surgery.
Definition
ChEBI: Pexidartinib is a pyrrolopyridine that is 5-chloro-1H-pyrrolo[2,3-b]pyridine which is substituted by a [6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridin-3-yl]methyl group at position 3. It is a potent multi-targeted receptor tyrosine kinase inhibitor of CSF-1R, KIT, and FLT3 (IC50 of 20 nM, 10 nM and 160 nM, respectively). Approved by the FDA for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT). It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an antineoplastic agent. It is a pyrrolopyridine, an organochlorine compound, an aminopyridine, an organofluorine compound and a secondary amino compound.
brand name
Turalio
General Description
Class: receptor tyrosine kinase; Treatment: TGCT; Other name: PLX3397; Elimination half-life = 26.6 h; Protein binding = >99%
Pharmacokinetics
Pexidartinib works by suppressing the growth of tenosynovial giant cell tumors. In clinical trials comprising of patients with symptomatic tenosynovial giant cell tumor, pexidartinib had a higher overall response rate, characterized by improved patient symptoms and functional outcomes, compared to placebo. Pexidartinib works by inhibiting the activation and signaling of tumor-permissive cytokines and receptor tyrosine kinases that play a central role in tumor cell proliferation and survival. Taking pexidartinib with a high-fat meal may increase the incidence and severity of adverse reactions, including hepatotoxicity.
Clinical Use
Pexidartinib is used for the treatment of adult patients with symptomatic tenosynovial giant cell tumour (TGCT) associated with severe morbidity or functional limitation not ameliorated by surgery.
in vitro
plx3397 strongly dampened the systemic and local accumulation of macrophages driven by b16f10 melanomas, without affecting gr-1+ myeloid derived suppressor cells [1].
in vivo
wild-type c57 mice were orthotopically injected with gl261 cells and fed with plx3397 compound. after 2 wks, tumors in the control group showed extensive microglia infiltration. in animals fed plx3397, however, there was a substantial reduction in the number of iba1- positive cells at the tumor [2].
Metabolism
Pexidartinib primarily undergoes oxidation mediated by hepatic CYP3A4 and glucuronidation by UGT1A4. Following UGT1A4-mediated glucuronidation, a major inactive N-glucuronide metabolite is formed with approximately 10% higher exposure than the parent drug after a single dose administration of pexidartinib. Based on the findings of in vitro studies, CYP1A2 and CYP2C9 may also play a minor role in drug metabolism.
storage
Store at -20°C
References
1)?DeNardo?et al.?(2011)?Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy;?Cancer Discov.?1?54 2)?Mok?et al.?(2014)?Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy;?Cancer Res.74?15 3)Sluijter?et al.?(2014)?Inhibition of CSF-1R supports T-cell mediated melanoma therapy;?PLoS One?9?e104230 4)?Peranzoni?et al.?(2018)?Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment;?Proc. Natl. Acad. Sci. USA?115?E4041 5)?Shi?et al.?(2019)?Modulating the Tumor Microenvironment via Oncolytic Viruses and CSF-1R Inhibition Synergistically Enhances Anti-PD-1 Immunotherapy;?Mol. Ther.?27?244 6) Dammeijer?et al.?(2017)?Depletion pf Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy;?Cancer Immunol. Res.?5?535
Properties of PLX3397 (Pexidartinib)
| Density | 1.458±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to at least 10 mg/ml) |
| pka | 12.81±0.40(Predicted) |
| form | solid |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 1029044-16-3 |
Safety information for PLX3397 (Pexidartinib)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PLX3397 (Pexidartinib)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1