Momelotinib
- CAS NO.:1056634-68-4
- Empirical Formula: C23H22N6O2
- Molecular Weight: 414.46
- MDL number: MFCD16038899
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
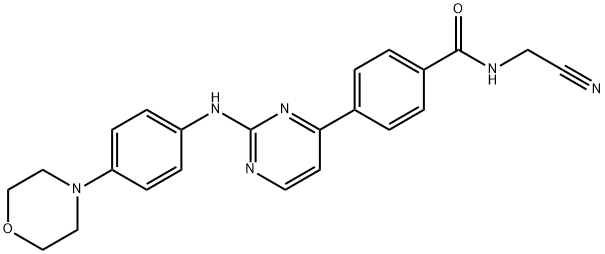
What is Momelotinib?
Absorption
Momelotinib is rapidly absorbed following oral administration with a bioavailability of 97%. The mean (%CV) steady-state Cmax is 479 ng/mL (61%), and the mean (%CV) AUC is 3,288 ng x h/mL (60%) at the maximum recommended dosage. Momelotinib exposure (i.e., Cmax and AUC) increases dose proportionally from 100 mg to 300 mg (0.5 to 1.5 times the maximum recommended dosage), but less than dose-proportional at doses from 400 mg to 800 mg (two to four times the maximum recommended dosage). There is no clinically significant accumulation. The Tmax at steady state is two hours (Q1: 1 hour; Q3: 3 hours) post-dose.
No clinically significant differences in momelotinib pharmacokinetics were observed following administration of either a high-fat meal (800 kcal; 50% fat) or low-fat meal (400 kcal; 20% fat) in healthy subjects.
Toxicity
No information is available regarding the LD50. There is no known antidote for overdose with momelotinib. If an overdose is suspected, the patient should be monitored for signs or symptoms of adverse reactions or effects, and appropriate supportive treatment should be instituted immediately. Further management should be as clinically indicated. Hemodialysis is not expected to enhance the elimination of momelotinib.
The Uses of Momelotinib
CYT387 inhibits Janus kinase 1 (JAK1) and Janus kinase (JAK2). These kinases are part of the JAK-STAT pathway and dysregulated functions are involved with hematology, oncology and inflammatory diseases.
Background
Momelotinib is a Janus Kinase 1 (JAK1) and 2 (JAK2) inhibitor. It is a competitive inhibitor of JAK ATP binding. First approved by the FDA on September 15, 2023, momelotinib is used to treat myelofibrosis. Myelofibrosis (MF) is a group of myeloproliferative neoplasms characterized by abnormal proliferative hematopoietic stem cells, leading to the release of cytokines and growth factors. MF includes primary MF (PMF), post-polycythemia vera (PV) MF, and post-essential thrombocythemia (ET) MF. Clinical manifestations of MF include anemia and thrombocytosis. Momelotinib works to block the JAK-signal transducer and activator of transcription (STAT) signalling pathway, which is aberrant in MF.
Indications
Momelotinib is indicated for the treatment of intermediate or high-risk myelofibrosis (MF), including primary MF or secondary MF [postpolycythemia vera (PV) and post-essential thrombocythemia (ET)], in adults with anemia.
What are the applications of Application
Cyt387 is an inhibitor of JAK1 and JAK2
Definition
ChEBI: Momelotinib is a benzamide obtained by formal condensation of the carboxy group of 4-{2-[4-(morpholin-4-yl)anilino]pyrimidin-4-yl}benzoic acid with the primary amino group of aminoacetonitrile. It is an ATP-competitive JAK1/JAK2 inhibitor with IC50 of 11 nM and 18 nM, respectively. Used for the treatment of patients with intermediate- or high-risk myelofibrosis. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, an antineoplastic agent, an anti-anaemic agent and an apoptosis inducer. It is an aminopyrimidine, a member of morpholines, a secondary amino compound, a tertiary amino compound, a member of benzamides and a nitrile.
Biological Activity
cyt387, an aminopyrimidine derivative discovered by high-throughput enzyme and cell-based screening along with the optimization using structure-guided medicinal chemistry, is a potent and selective inhibitor of janus kinase 1 (jak1), janus kinase 2 (jak2) and tyrosine kinase 2 (tyk2) with values of 50% inhibition concentration ic50 of 11 nm, 18 nm, 155 nm and 17 nm respectively. recent studies have revealed that cyt387, at low nanomolar concentrations ranging between 500 and 1500 nm, is able to inhibit jak2 signaling pathway, suppress proliferation and induce apoptosis in jak2-dependent hematopoietic cell lines with non-hematopoietic cell lines intact.tyner jw, bumm tg, deininger j, wood l, aichberger kj, loriaux mm, druker bj, burns cj, fantino e, deininger mw. cyt387, a novel jak2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. blood. 2010;115(25):5232-5240.
Pharmacokinetics
Momelotinib inhibits Janus Kinase 1 and 2 (JAK1/JAK2) with an IC50 of 11 and 18 nM, respectively. It also inhibits JAK3 (IC50 = 155 nM) and tyrosine kinase 2 (TYK2) (IC50 = 17 nM) with less selectivity. Momelotinib inhibited STAT3 phosphorylation in whole blood from patients with myelofibrosis (MF). Maximal inhibition of STAT3 phosphorylation occurred two hours after momelotinib dosing, which persisted for at least six hours. Iron availability and erythropoiesis were assessed by analysis of circulating hepcidin concentrations: an acute and sustained reduction of circulating hepcidin was observed for the duration of the 24-week administration of momelotinib to patients with MF.
Metabolism
Momelotinib is metabolized by multiple cytochrome P450 (CYP) enzymes, including CYP3A4 (36%), CYP2C8 (19%), CYP2C9 (17%), CYP2C19 (19%), and CYP1A2 (9%). M21 is initially formed via oxidation of the morpholine ring by the same CYP enzymes, followed by metabolism via aldehyde oxidase. M21 is a major metabolite in humans that retains approximately 40% of the pharmacological activity of the parent. The mean ratio of M21 to momelotinib for AUC ranged from 1.4 to 2.1. Momelotinib can undergo amide hydrolysis, N-dealkylation, nitrile hydrolysis, nitrile oxidation, and glucuronidation.
Properties of Momelotinib
| Density | 1.292 |
| storage temp. | Store at -20°C |
| solubility | insoluble in EtOH; insoluble in H2O; ≥20.7 mg/mL in DMSO |
| form | Orange powder. |
| pka | 10.94±0.46(Predicted) |
| color | Light yellow to yellow |
| CAS DataBase Reference | 1056634-68-4 |
Safety information for Momelotinib
Computed Descriptors for Momelotinib
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 CYT387 98% (HPLC) CAS 1056634-68-4View Details
CYT387 98% (HPLC) CAS 1056634-68-4View Details
1056634-68-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
