Pitavastatin calcium
- CAS NO.:147526-32-7
- Empirical Formula: C25H26CaFNO4
- Molecular Weight: 463.56
- MDL number: MFCD01937979
- EINECS: 807-641-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
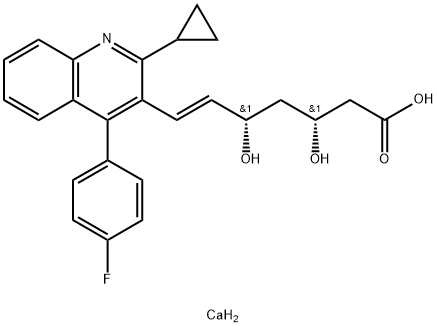
What is Pitavastatin calcium?
Description
Pitavastatin, launched for the treatment of hypercholesterolemia, belongs to the family of second-generation statins, also referred to as superstatins due to their improved efficacy as cholesterol lowering agents. Like other statins, pitavastatin reduces plasma cholesterol levels by competitively inhibiting HMG-CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis in the liver. It is a more potent inhibitor of HMG-CoA reductase than the previously marketed statins and has the potential benefit of not undergoing significant metabolism by CYP3A4. Pitavastatin is synthesized in a multi-step sequence, including the key step of introducing the dihydroxyheptenoate side chain by cross-coupling of a 3-iodoquinoline intermediate with an alkenylborane reagent. Unlike rosuvastatin, pitavastatin has a high oral bioavailability (~80%). Plasma protein binding is also high for pitavastatin (>95%), and regardless of the dosing, the highest tissue levels are found in the liver, its target organ. After oral administration, the peak plasma concentration is reached at ,0.8 h and the mean elimination half-life is ~11 h. Pitavastatin is only minimally metabolized, mainly by CYP2C8 and CYP2C9, and the predominant route of elimination of the parent drug and its metabolites is by means of bile excretion followed by elimination in the feces. In clinical studies, oral doses of 2–4 mg/day of pitavastatin produced dose-dependent reduction in LDL-cholesterol levels by 40–48% from baseline in patients with heterozygous familial hypercholesterolemia. In a 12-week double-blind comparative study, pitavastatin (2 mg/day) was more effective than pravastatin (10 mg/day) in reducing LDL-cholesterol levels (38 and 18%, respectively); however, both agents produced similar increases in HDL-cholesterol (~9%). The drug was well tolerated and the adverse reactions were mild and transient.
Chemical properties
White to Off-White Powder
Originator
Nissan (Japan)
The Uses of Pitavastatin calcium
Pitavastatin calcium (Livalo) is a novel member of the medication class of statins
The Uses of Pitavastatin calcium
A competitive inhibitor of HMG-CoA reductase. Antilipemic.
What are the applications of Application
Pitavastatin Calcium is a competitive inhibitor of HMG-CoA reductase
Definition
ChEBI: The calcium salt of pitavastatin. Used for treatment of hypercholesterolemia (elevated levels of cholesterol in the blood) on patients unable to sufficiently lower their cholesterol levels by diet and exercise.
brand name
Livalo
Hazard
A poison by ingestion.
Safety Profile
A poison by ingestion.Experimental reproductive effects. When heated todecomposition it emits toxic vapors of NOx and Fí.
Synthesis
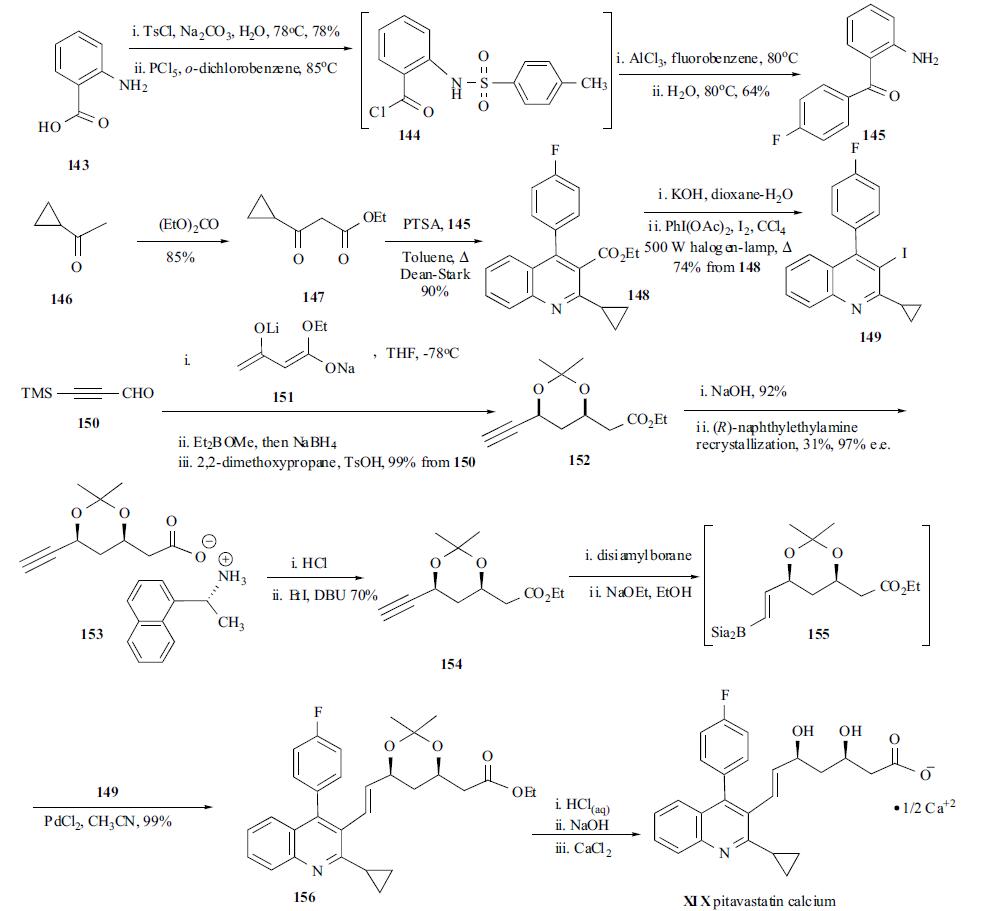
The convergent synthesis was achieved by cross-coupling of aryl halide 149 with (E)- alkenyl borane 155 which was derived from terminal acetylene 154 by via hydroboration. Anthranilic acid (143) was treated with TsCl and sodium carbonate in hot water to give N-tosylated intermediate in 78% yield, which was converted to the corresponding acid chloride 144 with PCl5 in o-dichlorobenzene at 85??C. Intermediate 144, without isolation, was reacted with fluorobenzene in the presence of AlCl3 at 80??C to give the Friedel-Crafts product which was then hydrolyzed in hot water to give fluorobenzophenone free aniline 145 in 64% yield from the N-tosyl anthranilic acid. Acetyl cyclopropane (146) was reacted with diethyl carbonate to give the corresponding ethyl ester 147. The quinoline core structure was obtained by condensing fluorobenzophenone 145 with 147 under acidic conditions with a Dean-Stark trap to give quinoline-3- carboxylic ethyl ester 148 in 90% yield. Ester 148 was hydrolyzed with potassium hydroxide, and the free carboxylic acid thus obtained was subsequently photoiododecarboxylated with iodine and PhI(OAc)2 to give aryl iodide 149 in 74% yield. 3-Trimethylsilylpropynal (150) was used as the starting material to prepare the chiral side chain. Compound 150 was reacted with di-anion 151 in THF at low temperature to give the corresponding diol ester which was first reacted with Et2BOMe and then reduced to acetylene with sodium borohydride. The free diol was protected as ketal with 2,2-dimethoxypropane in the presence of TsOH to give dimethylketal acetylene 152 in 99% yield. The ester functionality was hydrolyzed with sodium hydroxide to give the acid in 92% yield. The racemic free acid was resolved with (R)-(1-naphthyl)ethylamine to give the pure diastereomeric salt 153 which crystallized out in 31% yield and 97% e.e. Esterification of the free carboxylic acid liberated from the crystalline salt with ethyl iodide gave optically pure acetylene 154 in 70% yield. Hydroboration of acetylene 154 with disiamylborane gave (E)-alkenyldisiamylborane 155 and the excess borane reagent was quenched with sodium ethoxide in ethanol. After evaporation of all volatile material, the residue was directly subjected to the cross-coupling reaction. Palladium (II) chloride and aryl iodide 149 were mixed in acetonitrile to give coupling product 156 in 99% yield. After the ketal in 156 was hydrolyzed under acid conditions and the ester was hydrolyzed with sodium hydroxide, the resulting carboxylic sodium salt was reacted with calcium chloride to yield pitavastatin calcium (XIX) with 99% e.e.
storage
Desiccate at RT
Properties of Pitavastatin calcium
| Melting point: | >138oC (dec.) |
| alpha | D20 +23.1° (c = 1.00 in acetonitrile/water) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 147526-32-7(CAS DataBase Reference) |
Safety information for Pitavastatin calcium
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Pitavastatin calcium
| InChIKey | ADXNRJCYUMOXPZ-MNGIKBOWNA-N |
| SMILES | C(/C1C(C2CC2)=NC2C=CC=CC=2C=1C1C=CC(F)=CC=1)=C\[C@@H](O)C[C@@H](O)CC(=O)O.[Ca] |&1:22,25,r| |
Pitavastatin calcium manufacturer
SETV ASRV LLP
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
![(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester](https://img.chemicalbook.in/CAS/GIF/147489-06-3.gif)



You may like
-
 PITAVASTATIN CALCIUM 99%View Details
PITAVASTATIN CALCIUM 99%View Details -
 147526-32-7 99%View Details
147526-32-7 99%View Details
147526-32-7 -
 Pitavastatin calcium 98%View Details
Pitavastatin calcium 98%View Details
147526-32-7 -
 Pitavastatin calcium 147526-32-7 99%View Details
Pitavastatin calcium 147526-32-7 99%View Details
147526-32-7 -
 147526-32-7 Pitavastatin calcium 99%View Details
147526-32-7 Pitavastatin calcium 99%View Details
147526-32-7 -
 Pitavastatin calcium 98%View Details
Pitavastatin calcium 98%View Details
147526-32-7 -
 147526-32-7 PITAVASTATIN CALCIUM 95-99%View Details
147526-32-7 PITAVASTATIN CALCIUM 95-99%View Details
147526-32-7 -
 Pitavastatin calcium CAS 147526-32-7View Details
Pitavastatin calcium CAS 147526-32-7View Details
147526-32-7
